Ciara Gavin MPSI takes a clinical look at the prevalence, symptoms and treatment options for patients with rheumatoid arthritis and how pharmacists can help
Background
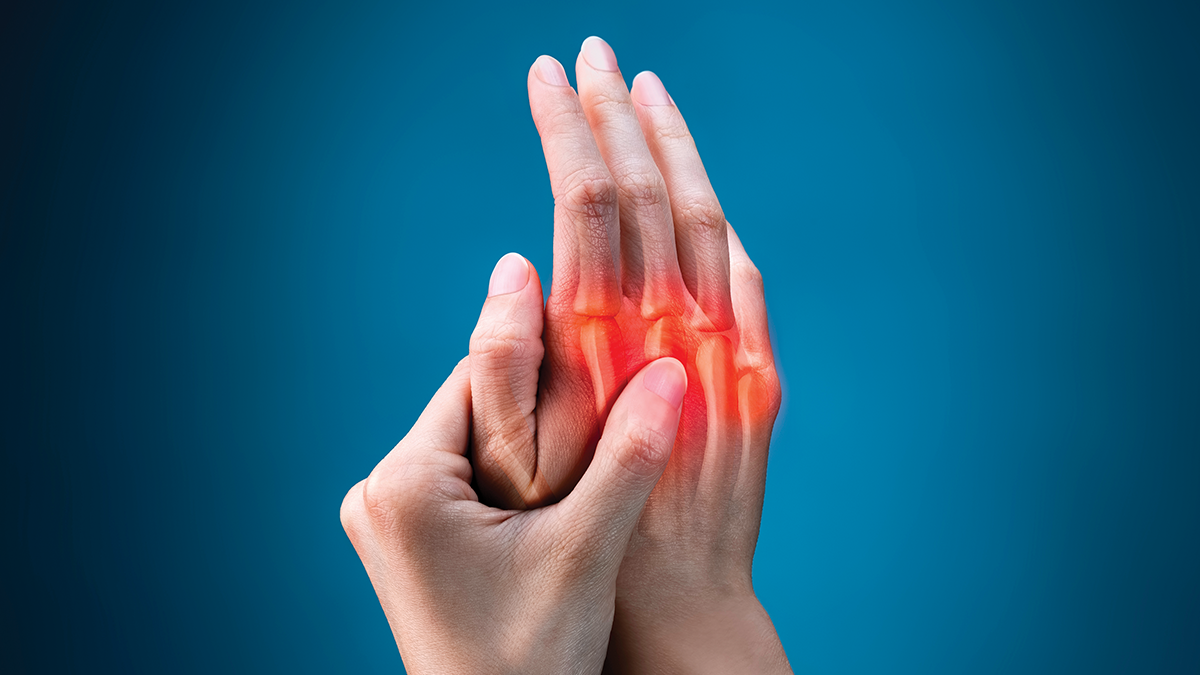
Rheumatoid arthritis is a common, chronic systemic inflammatory condition affecting around 1 per cent of the population.1 The condition manifests in patients as joint pain, stiffness and swelling.2 Rheumatoid arthritis (RA) primarily affects the small joints of the hands and feet.3 Due to the cartilage and bone destruction caused by untreated or progressed RA, it is recommended to commence treatment as soon as possible after diagnosis, and ideally within three months.2, 3, 4
Investigations
Laboratory tests
Once a clinical diagnosis is made, laboratory tests are ordered to help confirm diagnosis and determine prognosis:
? Rheumatoid factor (RF):3 This protein is produced by the immune system when it attacks healthy tissue and is identified in a laboratory test.5 RF is positive in 60-to-70 per cent of patients with RA. It is generally tested at first presentation or diagnosis and does not need to be repeated if positive. The higher values symbolise worse prognosis.6 About 5 per cent of patients without rheumatoid arthritis can also test positive for the protein.5
? Anti-CCP antibodies:3 Anti-cyclic citrullinated peptide antibody (anti-CCP) is a marker present in about 70 per cent of patients with RA.7 Those with anti-CCP positive laboratory tests are very likely to develop RA, but not everyone with RA will have anti-CCP antibodies present.
? Patients with both positive results for RF and anti-CCP antibodies are more likely to have severe RA.5
? ESR or CRP:3 Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are both non-specific laboratory tests which can measure inflammation levels in the body. These are often measured during RA diagnosis.5,8
Imaging
? X-rays:3 Scans of a patient’s affected joints are conducted to identify any erosion visible.
Assessing disease activity
? Disease activity measurement is important for assessing and identifying treatment plans for the patient. By quantifying disease activity, it is recognised as a tool for measuring treatment success also. There are several composite measures of disease activity developed and validated, but the most commonly used is the ’28 joint count disease activity scale’ – DAS28.4
? Scores of >5.1; >3.2 to ?5.1 or ?3.2 indicate the presence of high, moderate or low disease activity, respectively.4
Treatment
Treatment aims for RA are to control the symptoms and signs of RA and limit radiological damage; therapy should be started as soon as possible.4, 5
Steroids and non-steroidal anti-inflammatory drugs (NSAIDs) are used to control pain and the initial inflammatory process at the commencement of treatment9,3 and also can be used for acute flares during treatment.4
cDMARD: Conventional disease-modifying anti-rheumatic drugs monotherapy are first-line treatment.3 cDMARDs offered are: Methotrexate, leflunomide, sulfasalazine and hydroxychloroquine.3, 4, 5
Short–term glucocorticoids via oral, intramuscular or intra-articular administration can be used to bridge treatment when commencing a cDMARD.3
Methotrexate is often the cDMARD of choice first-line3, 4, 5 and is used either as monotherapy or in combination with another DMARD in approximately 70 per cent of RA treatment. Methotrexate is a folic acid antagonist and is classified as an antimetabolite cytotoxic agent.10 The usual starting dose is 7.5mg once a week, up-titrated to a maximum dose of 20mg once-weekly based on the patient’s response and haematological toxicity, as reviewed by patient’s blood tests.10 Due to the serious risk of toxicity associated with overdose of methotrexate, patients should be informed this is a ONCE-WEEKLY administration.
Patients should inform their doctor or pharmacist immediately if they experience any of the following symptoms:10, 11
? Sore throat/other infections.
? Fever/chills.
? Mouth ulceration.
? Easy bruising or bleeding.
? Diarrhoea.
? Vomiting.
? Unexplained rash.
? Breathlessness.
? Dry, persistent cough.
Due to the high-risk nature of the supply of methotrexate to patients in Ireland, The PSI published guidance for pharmacists which includes the following points:11
? The pharmacist must review the patient’s history of dispensed medication, including an assessment for time period since last dispensing and interaction review of medication. Only in exceptional circumstances should more than a month’s supply of methotrexate be dispensed at one time. If a patient presents to the pharmacy known to be taking methotrexate, they should be seen by the pharmacist, irrespective of the nature of the advice being sought.
? Product labelling must be clear for the patient. It is recommended to state the number of tablets, total dose, weekly interval and day of week on the dispensed medication, ie, take 6 x 2.5mg tablets (15mg in total) once a week on a Friday. The use of ‘as directed’ is not suitable.
? The dispensed product should always be double-checked.
? At the point of supply, the pharmacist should personally supply the methotrexate to the patient or carer, should verbally confirm the dose and reinforce the labelled instructions. The pharmacist must offer to counsel the patient every time a supply is made.
If a patient has not achieved the treatment target aim of remission or low disease activity despite dose escalation of single agent cDMARD, then a combination ‘step-up strategy’ is recommended where an additional cDMARD is commenced.3
If a patient has moderate-to-severe disease activity at initial presentation, or has not adequately responded to cDMARD alone, they are offered methotrexate plus a biological agent or targeted synthetic disease-modifying antirheumatic drug.
Biological agents
Biological agents, including TNF inhibitors, B-cell modulators, T-cell modulators and IL-6 inhibitors have improved the success of RA treatment and control in the recent era by modulating the inflammatory response.9
TNF-? is a cytokine involved in the inflammation process in RA. It has been found that the presence of TNF-? plays an extremely central role in the inflammation and bone degradation with RA,9 as it induces local inflammation and pannus formation, which leads to erosion of the cartilage and bone destruction. TNF-? inhibitors have expanded treatment options for RA. The TNF-? inhibitors block the chronic inflammation process.
TNF-? inhibitors used in the treatment of RA include:
? Etanercept: 50mg subcutaneously once-weekly or 25mg subcutaneously twice-weekly.
? Infliximab: 3mg/kg intravenous infusion at weeks 0, 2, 6, and then every eight weeks thereafter (the dose and/or frequency can be increased if incomplete response).
? Adalimumab: 40mg every two weeks subcutaneously.
? Golimumab: 50mg subcutaneously once monthly; or 2mg/kg intravenous infusion at weeks 0 and 4, and then every eight weeks thereafter.
? Certolizumab pegol: 400mg subcutaneously at weeks 0, 2, and 4, and then 200mg every two weeks or 400mg every four weeks thereafter.
IL-6 inhibitors
? Tocilizumab: 4mg/kg intravenous infusion every four weeks, may increase to 8mg/kg every four weeks if necessary, maximum 800mg/dose; body weight <100 kg: 162mg subcutaneously every two weeks initially, increase to 162mg once weekly if necessary; body weight ?100kg: 162mg subcutaneously once-weekly.
? Sarilumab: 200mg subcutaneously every two weeks.
T-Cell modulator
? Abatacept: Body weight <60 kg: 500mg intravenous infusion at weeks 0, 2, and 4, and then every four weeks thereafter; body weight 60-100kg: 750mg intravenous infusion at weeks 0, 2, and 4, and then every four weeks thereafter; body weight >100kg: 1,000mg intravenous infusion at weeks 0, 2, and 4, and then every four weeks thereafter.
B-Cell modulator
? Rituximab: 1,000mg intravenous infusion on days one and 15, may repeat course every 16-to-24 weeks if inadequate response.
Targeted synthetic DMARDs
JAK inhibitors: Janus kinase inhibitors block the intracellular enzyme that transmit signals from cytokines which drive the inflammatory cellular response in rheumatoid arthritis.
? Tofacitinib: 5mg orally (immediate-release) twice-daily.
? Baricitinib: 2mg or 4mg orally once-daily.
Upadacitinib: 15mg orally once-daily.
For further information on the management of rheumatoid arthritis, see the National Institute of Health and Care Excellence guidelines (2018).