Pharmacist and GP registrar Domhnall Heron provides an overview of medicines management updates in the context of the coronavirus pandemic
Since 1 April, the ICGP has held a weekly webinar updating GPs around various aspects of Covid-19. These have been immensely valuable and informative, with the clinical updates a particular highlight. Below are some clinical pearls from those webinars.
? HSE Covid-19: Interim Clinical Guidance — Immunosuppressant therapy
This very helpful document was published on 6 April 2020. It enables us to identify patients who may be at increased risk due to their regular immunosuppressant or their comorbidities. People are risk-stratified into normal risk (same risk as the general population), increased risk, and higher risk of contracting Covid-19.
Normal risk (same as general population)
? Those with an autoimmune disease not taking immunosuppressant therapies.
? Hydroxychloroquine.
? Mesalazine.
? Sulfasalazine.
? Penicillamine.
? Gold products.
? MABs, including reslizumab, benralizumab, mepolizumab, omalizumab, dupilumab.
(Although people on these medicines may be in high-risk groups for Covid-19 for other reasons, ie, severe respiratory disease.)
Increased risk
Any single medication below without prednisolone 5mg or greater in the last four weeks:
? Azathioprine.
? Mercaptopurine.
? Methotrexate.
? Leflunomide.
? TNF-alpha inhibitors, ie, adalimumab, etanercept, infliximab.
? Biologics for multiple sclerosis, ie, fingolimod, natalizumab.
? Interleukin inhibitors, including those for psoriasis.
? JAK inhibitors.
This list is not exhaustive. Please see HSE Covid-19: Interim Clinical Guidance — Immunosuppressant Therapy (6 April 2020).
Higher risk
? Prednisolone 40mg/day or greater for more than one week, or 20mg/day or greater for two weeks or longer.
? People taking two or more immunosuppressant medicines. This includes prednisolone 5mg or greater in the last four weeks.
? Cyclophosphamide or rituximab in the last six months.
? Poorly-controlled disease or a history of recurring infections while on immunosuppressants.
? People taking one immunosuppressant known to increase the risk of infection or serious infection and over 70 years of age/organ transplant/chemotherapy/severe respiratory conditions/pregnancy/significant heart disease.
Many patients take corticosteroids for various conditions. This document also advises on corticosteroid treatment not considered immunosuppressive and, therefore, not considered sufficient to significantly increase risk of infection.
Steroid treatment not considered immunosuppressive
? Short-term (less than seven days), irrespective of dose.
? Long-term (two weeks or greater), less than 20mg/day of prednisolone.
? Maintenance physiologic doses (replacement therapy).
? Fludrocortisone, less than 300 micrograms/day.
? Topical (skin or eyes) or by inhalation.
? Intra-articular, bursal, or tendon injection.
? Drug monitoring in primary care during Covid-19.
Some patients take medications that require regular blood monitoring. The Specialist Pharmacy Service from the UK’s NHS provides valuable guidance on the circumstances when blood monitoring may be safely extended.
Normal lithium monitoring includes checking lithium levels every three months for the first year then every three-to-six months. TFT, renal profile and weight check is every six months. Monitoring intervals can be extended by up to three months during Covid-19, unless in at-risk group (see Table 1).
A patient stable on a DMARD (methotrexate, azathioprine, leflunomide, mercaptopurine) is one who has been on current treatment for >12 months and at a stable dose for >six weeks. Normal monitoring is an FBC, renal profile, and LFTs every three months. Additionally, blood pressure and weight are to be checked at each monitoring visit for leflunomide. Consider extending the monitoring interval up to every six months in the absence of certain conditions.
Lithium at-risk patients — monitoring frequency not to be extended
? Elderly (>65 years).
? Have received <12 months’ treatment.
? Renal impairment (eGFR <60ml/min).
? Impaired thyroid function at last test.
? Raised calcium levels at last test.
? Poor symptom control or suspected poor adherence.
? Last serum lithium >0.8mmol/L.
? Introduction or removal of NSAIDs, ACEi, ARB and thiazide diuretics since last blood test.
Extending blood monitoring with DMARDs is not suitable if the patient has
? Poor renal function with CKD ?3.
? Severe liver disturbance or abnormal liver results due to DMARDs within previous three months.
? Severe abnormal WBC results due to DMARDs within previous three months.
? Hydroxychloroquine and azithromycin.
Hydroxychloroquine and azithromycin gained popularity as a treatment option early in the pandemic, especially after a French study from Marseille by Gautret et al was published on 17 March. This study claimed 100 per cent clearance of the virus in the subgroup taking both hydroxychloroquine and azithromycin (n=6). Some major flaws with the study included an underpowered sample size, absent medium- and long-term follow-up data, exclusion of those lost to follow-up and therefore no intention to treat analysis performed. Hydroxychloroquine and azithromycin are not recommended in primary care in the management of Covid-19.
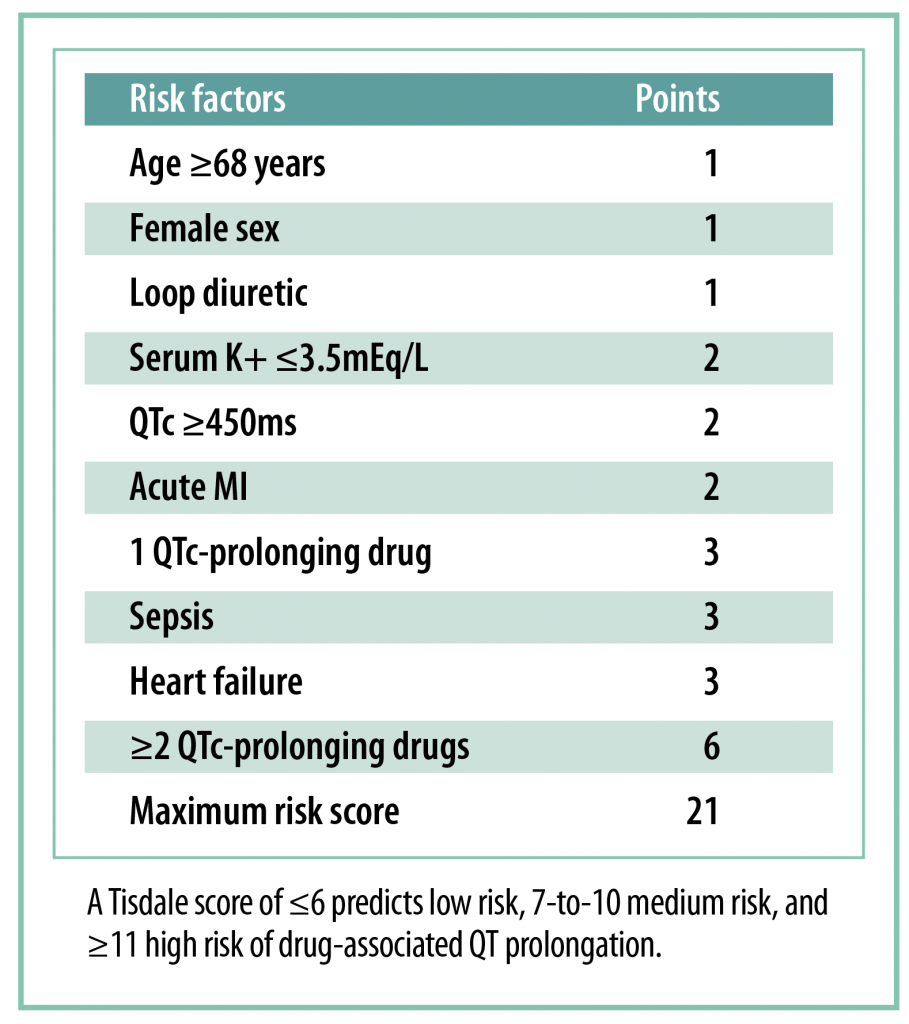
Both hydroxychloroquine and azithromycin can prolong the QT interval. Whenever being prescribed long-term, a baseline ECG and blood monitoring (renal profile and magnesium) are essential to calculate the ‘Tisdale Risk Score For Drug-Associated QTc Prolongation’. This was designed for inpatient care, but may be used as a guide in general practice.