Dr Tom Barrett, MB Dch MRCOG and Senior Medical Officer, HSE National Immunisation Office, provides clinical advice and an overview of the importance of flu vaccination for younger people in the year ahead
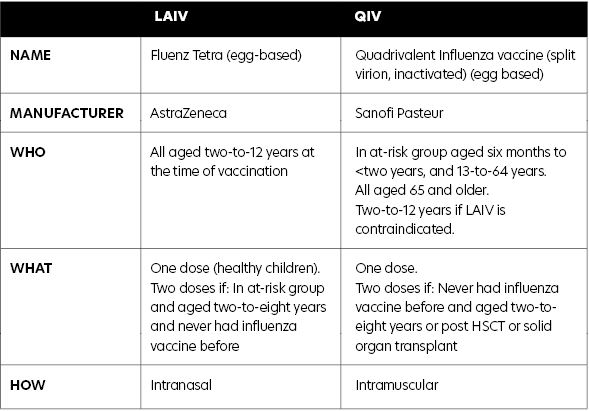
This year, the seasonal influenza vaccination programme has been extended to all children aged two-to-12 years. The aim of the programme is to prevent influenza and its complications in children and to reduce the spread of influenza to others in the community. The influenza vaccine offered to children is the quadrivalent live attenuated influenza vaccine (LAIV), which is given intranasally, and is available free of charge from GPs and community pharmacists.
Influenza disease
Influenza is a very common acute viral respiratory illness which affects all age groups. The virus is seen all-year-round but peaks every winter. The degree of influenza infection is unpredictable; however, each year in Ireland, influenza is responsible for between 200 and 500 deaths and as many as a 1,000 during a particularly severe season (such as 2008/2009).
The 2020/2021 influenza season presents a significant challenge to the delivery of healthcare services, both in primary care and in hospitals, especially if there is another resurgence of cases of Covid-19. At an individual level, the consequences of dual infection with influenza and Covid-19 are unclear, but worse outcomes are likely, particularly for those at-risk.
To prevent cases of influenza in children and reduce the spread of influenza to others, the National Immunisation Advisory Committee (NIAC) has recommended influenza vaccine for all children (aged two-to-17 years inclusive).
For the 2020/2021 influenza season, the Department of Health, on NIAC advice, has extended the influenza vaccination programme to children, providing funding to the HSE to offer quadrivalent LAIV vaccine free to all children aged two-to-12 years inclusive.
The aim of the extension of the influenza programme to children is to reduce:
- Morbidity and mortality from influenza in children.
- The number of people with influenza.
- The number of hospital admissions.
- Transmission of influenza to the elderly and persons in at-risk groups.
- Transmission to healthcare workers in families with children.
- Absenteeism of children from school and their parents from work.
Children outside of the two-to-12 age group (aged six months to <two years and aged 13 years and older) who have an underlying chronic medical condition should receive quadrivalent inactivated influenza vaccine (QIV), as in previous years.
It is estimated that 20-to-30 per cent of children develop influenza during each influenza season
Influenza in children
Children are among the most susceptible to influenza infection. It is estimated that 20-to-30 per cent of children develop influenza during each influenza season, compared to 5-to-10 per cent of adults. Children, because they have limited pre-existing immunity, are primary vectors of influenza transmission in the community and shed the virus at higher viral titres.
Children transmit the influenza virus for a longer period than adults; they can transmit the influenza virus for 10 or more days, compared to six days in adults, therefore increasing spread of the disease. Approximately 10 per cent of children under 15 attend their GP with influenza in an average influenza season. Influenza is an important cause of pneumonia, bronchitis, otitis media, croup and bronchiolitis in children. Incidence rates are highest in the younger age groups, leading to high rates of excess outpatient visits, hospital admissions and antibiotic prescriptions.
In Ireland during the 2018/2019 influenza season, 1,245 children were hospitalised with influenza. Children aged less than five years had the second-highest hospitalisation rates for influenza after those aged 65 years and older.
Between the 2009/2010 and 2018/2019 influenza seasons:
- 4,750 children aged 0-to-14 have required hospitalisation as a result of influenza, with
- 183 requiring critical care, and
- 41 children died.
Influenza vaccination for children in other countries
Nine European countries, the US, Canada and Australia currently recommend influenza vaccination for children. The UK gives LAIV to children. Finland, the US and Canada give LAIV or QIV to children. In the US, LAIV has been recommended since 2004. LAIV has been authorised for use in Canada since 2011.
In 2013, the UK introduced trivalent LAIV for two- and three-year-olds, with pilot programmes for primary school children. Quadrivalent LAIV was introduced during the 2014/2015 influenza season. The programme has extended year-on-year to include all children from two up to 11 years.
In Finland, annual inactivated influenza vaccine was recommended for children aged six-to-35 months in 2007. LAIV was introduced in 2015 to enhance vaccine uptake. Since then, all two- and three-year-old children have been eligible for vaccination with either LAIV or inactivated influenza vaccine. The programme has recently been extended to include all children to six years of age.
In Ireland, for the 2020/2021 influenza season, vaccination of children will help minimise the burden of influenza by preventing influenza in children and the transmission of influenza from children to those in at-risk groups. This should reduce morbidity from influenza as well as influenza-related hospital admissions in all the at-risk groups.
This is especially important this coming influenza season to minimise the impact on our health services from dual outbreaks of influenza and Covid-19.
LAIV for 2020/2021 influenza season
The vaccine used is quadrivalent LAIV Fluenz Tetra, manufactured by AstraZeneca, which will be offered to all children aged two years to 12 years. It is administered intranasally.
The vaccine contains the following four vaccine virus strains, as recommended by the WHO:
- An A/Guangdong-Maonan/SWL1536/2019 (H1N1)pdm09-like virus.
- An A/Hong Kong/2671/2019 (H3N2)-like virus.
- A B/Washington/02/2019 (B/Victoria lineage)-like virus.
- A B/Phuket/3073/2013 (B/Yamagata lineage)-like virus.
LAIV may contain residues of egg proteins (ovalbumin), maximum amount of less than 0.024 micrograms, and gentamicin. LAIV does not contain thiomersal or latex.
LAIV effectiveness
Since LAIV contains live attenuated viruses, it mimics natural infection with wild-type viruses, with the development of both mucosal and systemic immunity. Local mucosal antibodies protect the upper respiratory tract and may be more important for protection than serum antibodies, inducing more durable immune memory and so providing better long-term protection to children than inactivated influenza vaccines such as QIV.
In some studies, LAIV has been shown to be more effective in children compared with inactivated influenza vaccines. In addition, LAIV may offer some protection against strains not contained in the vaccine, as well as virus strains that have undergone antigenic drift.
A recent meta-analysis of LAIV suggested an efficacy against confirmed disease of 83 per cent (95 per cent CI 69-91 per cent).
The UK pilot primary school programme introducing LAIV was evaluated in 2014/2015 and showed:
- 94 per cent reduction in primary school-age children GP influenza-like consultations.
- 74 per cent reduction in primary school-age emergency department attendances with respiratory complaints.
- 93 per cent reduction in primary school-age confirmed influenza hospitalisations.
- 59 per cent reduction in adults’ GP influenza-like illness consultations.
LAIV dose and administration
Each LAIV vaccine comes as a pre-filled nasal applicator and each applicator contains 0.2ml nasal suspension. The vaccine is administered by the nasal route. One dose of LAIV is 0.2ml administered in divided doses into each nostril, ie, 0.1ml in each nostril.
If the child’s nose drips after vaccination, the vaccine dose does not need to be repeated. The vaccine is immediately absorbed after administration. Parents and guardians should be reassured the vaccine is still effective if this occurs. For the same reason, the vaccine does not need to be repeated if the child sneezes or blows their nose after vaccination.
LAIV can be given together with or at any time before or after the administration of
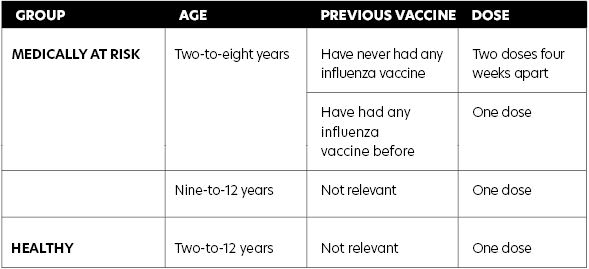
any other live attenuated (ie, MMR) or inactivated vaccines. NIAC has recommended healthy children require one dose of LAIV. However, children in a medically at-risk group aged two-to-eight years inclusive are recommended two doses of LAIV if they have never had any influenza vaccine before (see Table 1). The two doses should be given four weeks apart.
Contraindications to LAIV
- Anaphylaxis following a previous dose of influenza vaccine or any of its constituents (other than ovalbumin — see precautions).
- Asthma.
- Acute exacerbation of symptoms, increased wheezing and/or additional bronchodilator treatment in the last 72 hours.
- Seek specialist advice if on regular oral steroids or previous ICU admission.
- Significant immunosuppression due to disease or treatment.
- Children who live with severely immunosuppressed persons (ie, post haematopoietic stem cell transplant (HSCT)).
- Concomitant use of aspirin/salicylates.
- Influenza antiviral medication within the previous 48 hours.
- Those with severe neutropaenia (absolute neutrophil count <0.5 × 109/L) to avoid an acute vaccine-related febrile episode.
- Those on combination checkpoint inhibitors (ie, ipilimumab plus nivolumab) because of a potential association with immune-related adverse reactions.
The following are NOT contraindications:
- Asymptomatic HIV infection.
- Children receiving:
- Topical or inhaled corticosteroids.
- Low-dose systemic corticosteroids.
- Replacement therapy corticosteroids(ie, adrenal insufficiency).
Precautions
- Defer until recovered from an acute severe febrile illness.
- As LAIV has an ovalbumin content <0.1 micrograms per dose, it can be given to children with confirmed egg anaphylaxis or egg allergy in a primary care setting, except children who have required ICU admission to hospital for a previous severe anaphylaxis to egg, who should be given LAIV in hospital.
- Aspirin/salicylates should not be used for four weeks after vaccination unless medically indicated, as Reye’s syndrome has been reported following the use of salicylates during wild-type influenza infection.
- Avoid influenza antiviral medication for two weeks post-vaccination.
- Children aged two-to-12 years for whom LAIV is contraindicated should be offered QIV (provided there are no contraindications to QIV).
Gelatin in LAIV
LAIV, like some other vaccines, contains gelatin derived from pork, which is highly purified and hydrolysed and acts as a stabiliser. Gelatin in vaccines may cause concern to some members of the Muslim community. The National Immunisation Office (NIO) has received a statement from the Chair of the Irish Council of Imams stating that vaccines containing gelatin are permitted. See www.immunisation.ie for further details.
LAIV side-effects
Very common or common (more than one-in-10 to one-in-100): Nasal congestion/rhinorrhoea, decreased appetite, malaise, fever, headache, and myalgia. In post-marketing surveillance, overall rates of fever were similar to the rates following other childhood vaccines and were generally mild and of short duration.
Very rare (less than one-in-10,000):
Immediate allergic reactions
Very rare cases of Guillain-Barré syndrome (GBS) have been observed in the post-marketing setting following influenza vaccination. The risk of GBS following influenza infection is significantly greater than that following influenza vaccination.
Can LAIV vaccine cause virus shedding?
The attenuated vaccine viruses in LAIV are cold-adapted. They can replicate at the lower temperatures found in the nose, but cannot replicate efficiently at body temperature elsewhere in the body. Vaccinated children can shed the attenuated virus for a few days after vaccination but the virus that is shed cannot cause infection. Peak incidence of shedding occurred two-to-three days post-vaccination in Fluenz clinical studies.
Each chapter of NIAC immunisation guidelines advise:
“In some circumstances, advice in these guidelines may differ from that in the Summary of Product Characteristics (SmPC). When this occurs, the recommendations in these guidelines, which are based on current expert advice from NIAC, should be followed.”
NIAC advice, based on the current expert guidance, supersedes the advice in the licensed SmPC.
NIAC guidelines advise that “children who live with severely immunosuppressed persons requiring isolation (ie, post HSCT) should not receive the Quadrivalent Live Attenuated Influenza (LAIV) nasal vaccine”. This is a precautionary measure.
Therefore, NIAC advice is that LAIV vaccine can be given to any child for whom the LAIV vaccine is not contraindicated, who is living with any other person unless the adult is in isolation, ie, following a HSCT.
Children who are vaccinated with LAIV can ‘shed’ very small amounts of the weakened virus that is in the vaccine for a few days after vaccination. But the weakened viruses do not cause flu infection in others, or in the person vaccinated.
NIAC makes no recommendation that children avoid other vulnerable people, including a parent who is on chemotherapy or immunotherapy. NIAC advises: “Millions of doses of LAIV have been administered in the US for over 10 years and serious illness amongst immunocompromised contacts inadvertently exposed to vaccine virus has never been observed.”
Increasing uptake of influenza vaccine
Research, both in Ireland and elsewhere, has consistently shown that doctors and other healthcare professionals are the most trusted sources of information on vaccination. A recommendation by a trusted healthcare
professional has been shown to increase vaccine uptake.
Reminders to patients about vaccination have also been shown to increase vaccine uptake, be that by text, phone, email or letter. This influenza season, your recommendation to get the influenza vaccine to parents of children and other at-risk groups will be even more important in order to maximise uptake.
Summary
The introduction of the LAIV vaccine for children will help minimise the burden of influenza by protecting children from influenza and preventing transmission of influenza from children to those in at-risk groups. The NIO has developed an e-learning module for vaccinators, available at www.HSEland.ie.
The www.immunisation.ie website is updated with details on the LAIV vaccine. The NIO has produced information materials on LAIV for parents and for vaccinators and copies have been distributed to vaccinators, with additional copies available to order.
Influenza remains a major public health issue and never more so than during the Covid-19 pandemic. Influenza vaccination is the best intervention available. Extending the influenza vaccination programme to children aged two-to-12 years aims to protect children and reduce transmission to others, thereby reducing the burden on our health services at this crucial time.
Contraindications
- Anaphylaxis following a previous dose of influenza vaccine or any of its constituents (except ovalbumin).
- Severe neutropaenia (absolute neutrophil count less than 0.5 x 109/l)
- On combination checkpoint inhibitors (ie, ipilimumab plus nivolumab).
- Asthma — acute exacerbation of symptoms, increased wheezing and/or additional bronchodilator treatment in the last 72 hours.
- Seek specialist advice if on regular oral steroids or previous ICU admission.
- Concomitant use of aspirin/salicylates.
- Influenza antiviral medication in the previous 48 hours.
- Pregnancy.
- Significant immunosuppression due to disease or treatment.
- Those who live with severely immunosuppressed persons (ie, post HSCT).
Precautions
- Acute severe febrile illness; defer until recovery.
- Children who required ICU admission for a previous severe egg anaphylaxis should be given LAIV in hospital.
- No aspirin/salicylates for four weeks after vaccine due to risk of Reye’s syndrome.
- Avoid antiviral medication for two weeks after vaccine.
- Seek specialist assessment for those who required ICU admission for a previous severe egg anaphylaxis.
- Separate QIV from PCV vaccine by at least one week for children aged 12-to-23 months.
Adverse reactions
Very common or common:
Nasal congestion/ rhinorrhoea, decreased appetite, malaise, fever, headache and myalgia. (Fever rates similar to those after other childhood vaccines; generally mild and of short duration.)
Very common: Injection site pain and swelling. Fever, fatigue, myalgia, and irritability in young children.
Common: Drowsiness, sweating and arthralgia.
Very rare: Immediate allergic reactions. Guillain-Barré syndrome (risk of GBS following infection is much greater than that post vaccination).
The following resources on LAIV are available from the NIO:
- FAQs for healthcare professionals: https://www.hse.ie/eng/health/immunisation/pubinfo/flu-vaccination/faqchild.pdf.
- Algorithms on flu vaccination for children for healthcare professionals: https://www.hse.ie/eng/health/immunisation/pubinfo/flu-vaccination/laivalgorithm.pdf.
- Information materials for parents in different languages and easy-read guides: https://www.hse.ie/eng/health/immunisation/pubinfo/flu-vaccination/information/.
- Commonly-asked questions and answers on the safety of LAIV for parents: https://www.hse.ie/eng/health/immunisation/pubinfo/flu-vaccination/flu-vaccine-for-children/fluvaccineqas.html.