Dr Apama Keegan outlines the options for vaccination as winter approaches, with flu and Covid-19 posing a double threat to public health
For the first time, influenza (flu) vaccination rates in Ireland for people aged 65 years and older reached the WHO target of 75 per cent during the 2021/2022 season. This is a significant milestone for Ireland and is thanks to the concerted effort of all who plan and deliver the flu vaccine programme. Re-vaccination is recommended every flu season because circulating strains of the influenza virus change and immunity declines over time after vaccination.
Influenza is a very common acute viral respiratory illness which affects all age groups. The virus is seen all year round, but peaks every winter. The degree of influenza infection is unpredictable, however — each year in Ireland, influenza is responsible for between 200 and 500 deaths.
Although there was no flu circulating in Ireland during 2020/2021 due to public health restrictions in place because of the Covid-19 pandemic, flu activity returned in Ireland and across Europe during the 2021/2022 season as restrictions eased.
This winter, the 2022/2023 flu season presents a significant challenge to the delivery of healthcare services both in primary care and in hospitals, as flu and Covid-19 are expected to co-circulate.
The World Health Organisation (WHO) latest surveillance report states: “Countries are recommended to prepare for the co-circulation of influenza and SARS-CoV-2 viruses. They are encouraged to enhance integrated surveillance to monitor influenza and SARS-CoV-2 at the same time, and step-up their influenza vaccination campaign to prevent severe disease and hospitalisations associated with influenza. Clinicians should consider influenza in differential diagnosis, especially for high-risk groups for influenza, and test and treat according to national guidance.”
Each year, influenza statistics reinforce that flu is more severe in older adults, those with long-term heart or respiratory illness, in young children, and those who are pregnant. Eight-to-nine out of every 10 flu-related deaths occur in older adults, therefore it is important that older adults are protected early every flu season.
INFLUENZA VACCINES AVAILABLE FOR 2022/2023 SEASON
The best way to prevent Influenza is by vaccination. Each year, the WHO recommends the vaccine compositions based on the four virus strains most likely to be circulating in the coming season. The 2022/2023 HSE seasonal vaccination programme is offering three vaccines:
- Quadrivalent live attenuated influenza vaccine (LAIV), nasal application for children aged two-to-17 years.
Brand available:
- Fluenz Tetra nasal spray suspension influenza vaccine (live attenuated, nasal) manufactured by AstraZeneca AB.
- Inactivated quadrivalent influenza vaccine (QIV) available for all other eligible populations, including those aged two-to-17 years with contraindications to LAIV (QIV is licensed for those six months of age and older). Two brands of injectable vaccine will be distributed and are interchangeable where two doses are required:
- Quadrivalent Influenza Vaccine (split virion, inactivated) manufactured by Sanofi Pasteur.
- Influvac Tetra marketed by Mylan.
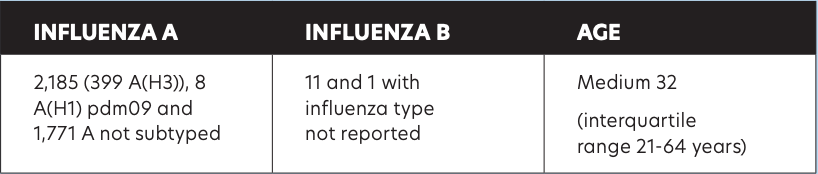
Table 1: Total of 2,197 influenza cases in Ireland for 2021/2022 (between week 40 2021 and week 20 2022)
RECOMMENDED GROUPS FOR INFLUENZA VACCINATION
The National Immunisation Advisory Committee recommends annual influenza vaccination for the following groups:
- Aged 65 years and older.
- Aged two-to-17 years.
- Healthcare workers.
- Pregnant women.
- Those living in a nursing home or other long-term care facility.
- Those in regular contact with pigs, poultry or waterfowl.
People with the following conditions can also get a free flu vaccine:
- Chronic heart disease, including acute coronary syndrome.
- Chronic liver disease.
- Chronic kidney failure.
- Chronic respiratory disease, including chronic obstructive pulmonary disease (COPD), cystic fibrosis, moderate or severe asthma, or bronchopulmonary dysplasia.
- Chronic neurological disease including multiple sclerosis, hereditary and degenerative disorders of the central nervous system.
- Diabetes.
- Down syndrome.
- Haemoglobinopathies.
- A body mass index (BMI) over 40.
- Immunosuppression due to disease or treatment (including asplenia or hyposplenism, and all cancer patients).
- Children with a moderate-to-severe neurodevelopmental disorder such as cerebral palsy.
- Children on long-term aspirin therapy.
- Any condition that can compromise respiratory function, like spinal cord injury, seizure disorder or other neuromuscular disorder, especially people also attending special schools or day centres.
Free flu vaccines can be offered to carers or household contacts of people who have:
- A health condition listed above.
- Down syndrome.
People eligible for the HSE flu vaccine should not be charged an administration fee or be charged for the HSE flu vaccine.
Children are among
the most susceptible to
influenza infection and
are twice as likely as
adults to catch the virus
INFLUENZA VACCINE UPTAKE IN AT-RISK GROUPS
Significant improvements have been made in recent years to improve vaccination uptake, notably in older adults, with a 75.4 per cent uptake recorded among people aged 65 years and older for the 2021/2022 season. However, vaccination rates for other at-risk groups, particularly children, remain low and have yet to reach the WHO target of 75 per cent. Therefore it is important to encourage uptake of the influenza vaccine this flu season.
Those most at risk from flu are dependent on their health providers, nurses, doctors and pharmacists for information on influenza vaccination. A recommendation by a trusted healthcare professional for their patients who are at an increased risk of influenza-related complications to attend their GP or pharmacist for vaccination has been shown to increase vaccine uptake.
KEY FOCUS FOR 2022/2023 SEASON – CHILDREN AGED TWO-TO-17 YEARS
The nasal spray flu vaccine is recommended for all children aged two-to-17 years. The nasal flu vaccine protects children from flu-related morbidity and mortality.
Children are among the most susceptible to influenza infection and are twice as likely as adults to catch the virus. Approximately 10 per cent of children under 15 attend their GP with influenza-like illness in an average influenza season. Influenza in children can cause serious complications such as pneumonia, bronchitis, otitis media, croup, and bronchiolitis.
Children attending crèches, daycare centres and schools are important transmitters of influenza in the community. This means that vaccinating children decreases the number of people with influenza, the number of hospital admissions, transmission of influenza to older adults and persons in at-risk groups, and transmission to healthcare workers in families with children.
Children also transmit the flu virus for a longer period than adults; they can transmit the virus for 10 or more days, compared to six days in adults, therefore increasing the spread of the disease, increasing the pressure on the healthcare system, and contributing significantly to influenza outbreaks.
A meta-analysis of LAIV in 2012 suggested an efficacy against confirmed disease of 83 per cent (95% CI 69-91%) in younger children.
Vaccinating children not only protects them against severe illness, but may also assist in reducing influenza transmission to other vulnerable populations, such as those aged 65 years and older and those with long-term health conditions.
KEY FOCUS FOR 2022/2023 SEASON – HEALTHCARE WORKERS
Healthcare staff are up to 10 times more likely to get influenza compared to the general population. It is estimated that one-in-five healthcare workers are infected with flu every year and many continue to work despite being ill, which increases the risk of spread of influenza to their colleagues and patients.
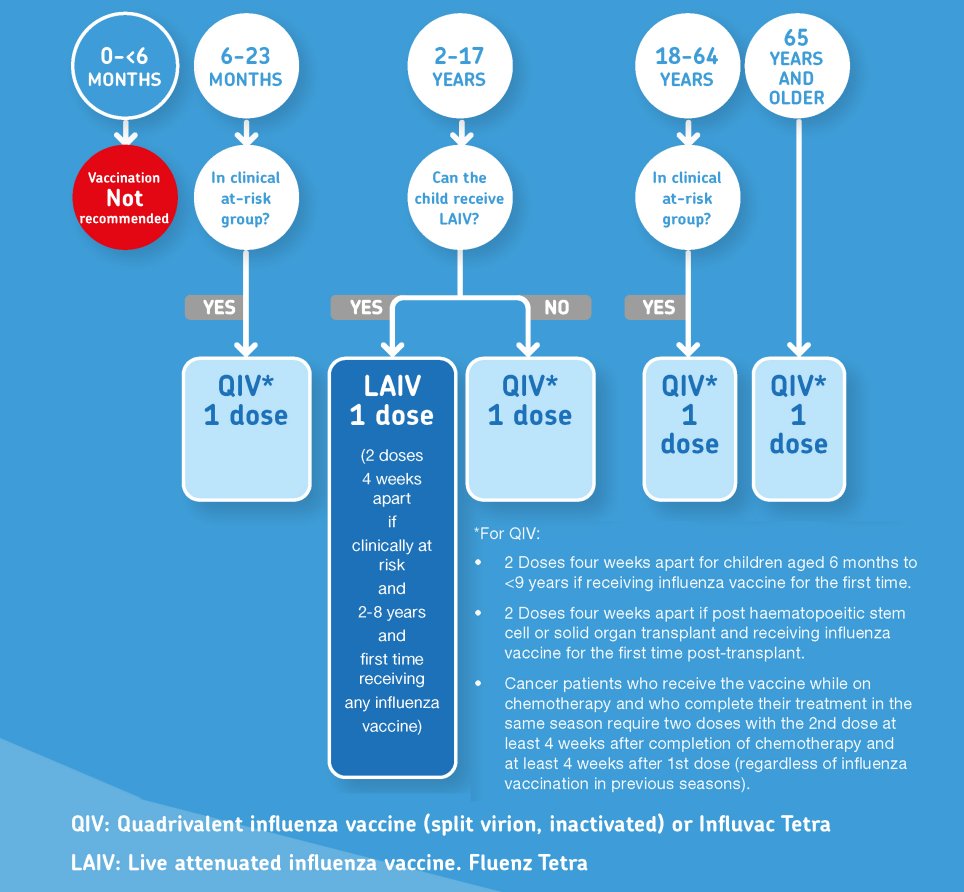
Figure 1: Algorithm for use of the two types of flu vaccines by age
Because the influenza virus is transmissible before symptoms develop, many healthcare workers transmit the disease to their high-risk patients. During hospitalisation, patients in general are five-to-35 times more likely to acquire influenza if exposed to infected patients or healthcare workers. At-risk patients rely on healthcare workers to be vaccinated for their own protection, as some cannot generate an adequate immune response from vaccination themselves.
Flu vaccination of healthcare workers results in an up to 40 per cent reduction in influenza-related patient deaths. Reduced rates of influenza-like illness, hospitalisation and deaths from influenza in older adults, and a reduction in healthcare worker sick leave have also been observed in institutions with high levels of healthcare worker immunisation in Europe.
This flu season, HSE healthcare workers can avail of their free flu vaccine from peer vaccinators in their workplace or from participating GPs and pharmacies. It is recommended that all staff in general practice get their flu vaccine this season too.
While rates of vaccine uptake among healthcare workers in Ireland have improved in recent years, the latest uptake rates of healthcare workers in public hospitals saw a reduction from 71.0 per cent in 2020/2021, to 64.0 per cent for the 2021/2022 influenza season.
Achieving higher healthcare worker vaccination uptake is more important now than ever in preventing the spread of influenza and the double threat of flu and Covid-19 on healthcare services. Leadership by senior medical and nursing staff has shown to be a key factor in better uptake rates.
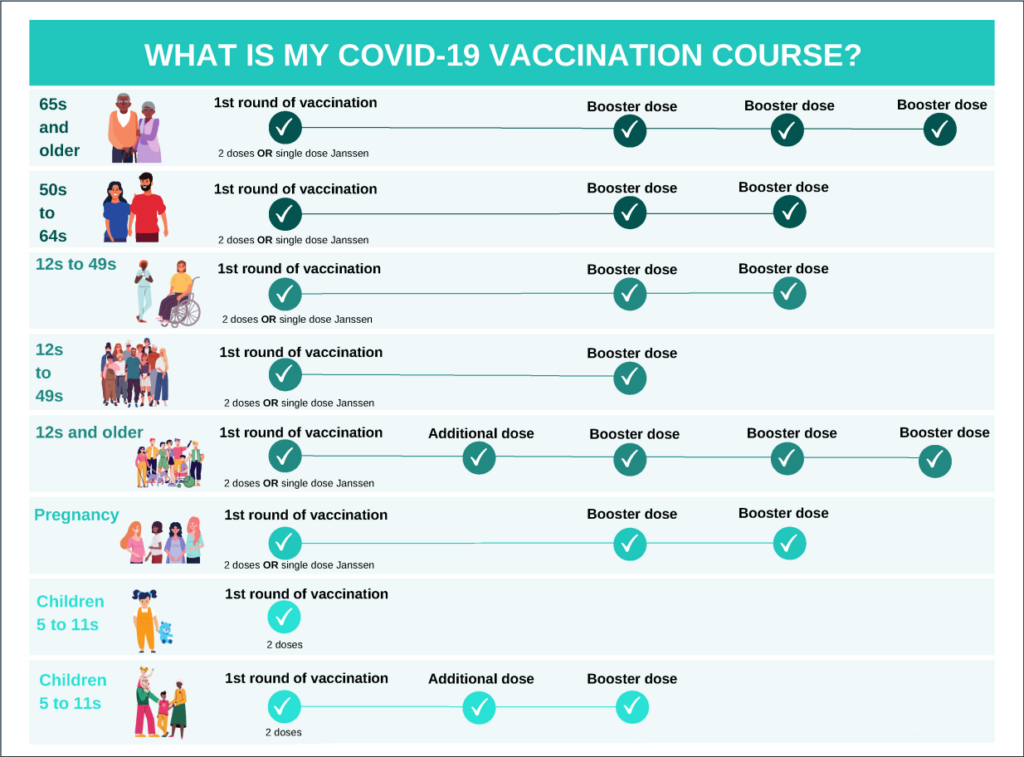
Figure 2: Recommended Covid-19 vaccination course for people living in Ireland by NIAC
CO-ADMINISTRATION OF INFLUENZA VACCINE WITH OTHER VACCINES
The National Immunisation Advisory Committee (NIAC) has advised that all influenza vaccines and other vaccines (except PCV13 for children aged 12-to-23 months) may be administered at the same time or at any interval.
INFLUENZA AND COVID-19
The influenza vaccine and Covid-19 vaccines can be administered at the same time to eligible groups. If they are getting a Covid-19 booster, there should be an interval of at least four months since the last Covid-19 vaccine or Covid-19 infection (post completed primary Covid-19 vaccination course).
As it is not known if Covid-19 vaccine reactogenicity is increased with coadministration, the flu vaccine and Covid-19 vaccines should be preferably given in different limbs. It is recommended that when people get vaccinated, they are informed that there may be a slight increase in short-term mild adverse events after co-administration of the flu vaccine and a Covid-19 vaccine. These can include pain at the site of injection, fatigue, headache, and myalgia.
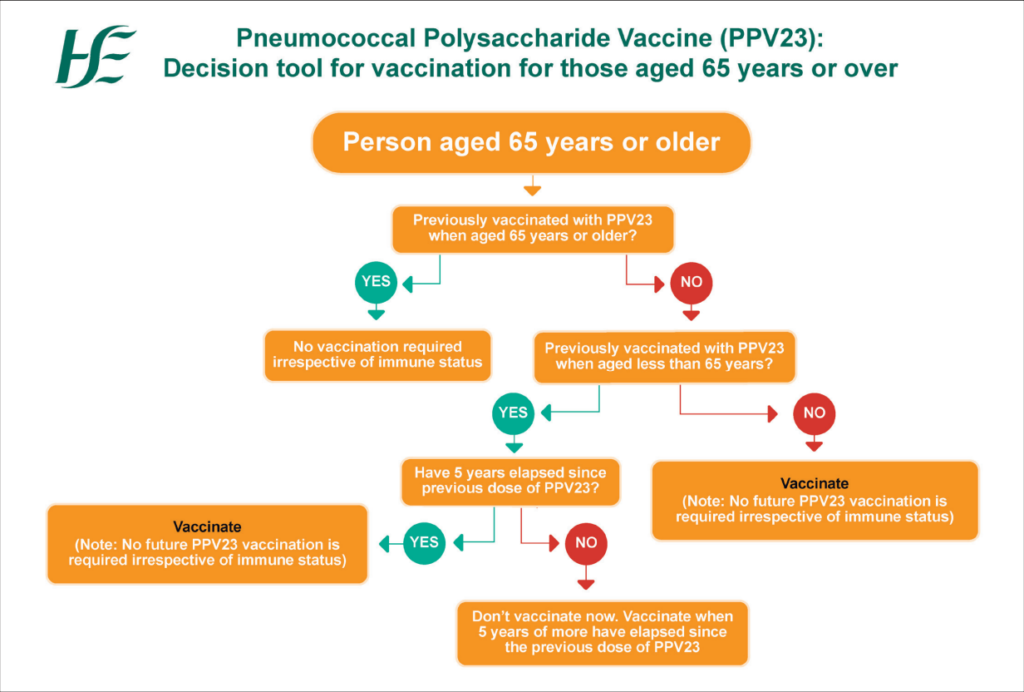
Figure 3: Algorithm for PPV23 for people aged 65 and over
As influenza and Covid-19 are caused by two different viruses, it is important for eligible groups to get both vaccines.
This winter, the HSE is encouraging those eligible — especially anyone aged 50 or older who is yet to get their Covid-19 booster, and those aged 65 years and older for their next booster and flu vaccine, healthcare workers, children, people with certain long-term conditions, and anyone pregnant — to top-up their immunity by getting their Covid-19 booster and flu vaccine to make sure they are protected in the months ahead.
Using available mRNA Covid-19 vaccines, NIAC advises that the following groups are recommended a Covid-19 booster:
1. A third mRNA Covid-19 booster vaccine is recommended for:
- Those aged 65 years and older (fifth Covid-19 vaccine dose).
- Those aged 12-to-64 years with immunocompromise associated with a suboptimal response to vaccines at the time of their primary or booster vaccination (sixth Covid-19 vaccine dose). When practicable, these booster doses of Covid-19 vaccine and influenza vaccine can be given at one visit.
When pneumococcal bacteria spread from the nose and throat to the ears or sinuses, it typically causes mild infections
2. A second mRNA Covid-19 booster vaccine is now recommended for:
- Those aged 50-to-64 years (fourth Covid-19 vaccine dose).
- Those aged 12-to-49 years who have underlying medical conditions associated with a higher risk of severe Covid-19, ie, diabetes (fourth Covid-19 vaccine dose).
- Those aged 12-to-49 years with underlying medical conditions who are residents of long-term care facilities (fourth Covid-19 vaccine dose).
3. A second mRNA Covid-19 booster vaccine is recommended for:
- Healthcare workers, and when practicable, this booster dose can be given at the same time as the influenza vaccine (fourth Covid-19 vaccine dose).
4. To enhance maternal protection and provide optimal benefit to the infant, an additional mRNA Covid-19 booster vaccine is recommended in pregnancy at 16 weeks’ gestation or later for those who have not received a booster vaccine in the current pregnancy (fourth Covid-19 vaccine). This timing is to enhance protection to the mother and the infant.
5. A first mRNA Covid-19 booster vaccine is now recommended for those aged five-to-11 years with immunocompromise associated with a suboptimal response to vaccines at the time of their primary or additional vaccination (third Covid-19 vaccine).
It is important to follow the recommended Covid-19 vaccine journey for each patient (See Figure 2).
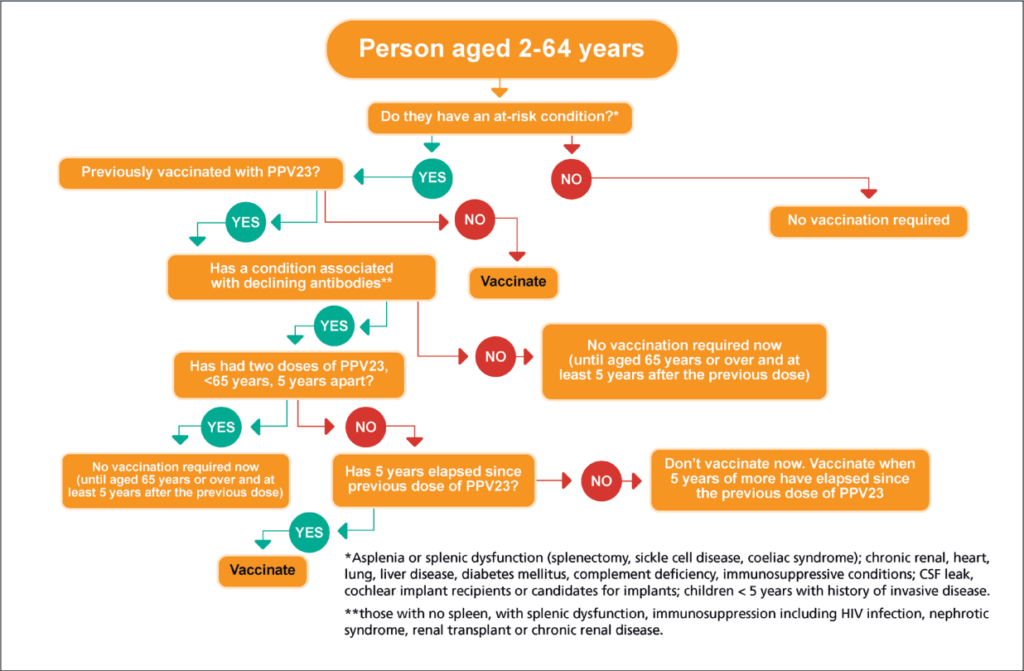
Figure 4: Algorithm for PPV23 for people aged two-to-64 years old
PNEUMOCOCCAL POLYSACCHARIDE VACCINE (PPV23)
The pneumococcal polysaccharide vaccine, PPV23 (Pneumovax) can also be administered to eligible groups at the same time or at any interval as the influenza and Covid-19 vaccines.
Pneumococcal disease is a serious, but vaccine-preventable disease. It is caused by the common bacteria, Streptococcus pneumoniae. Like influenza, pneumococcal bacteria can spread from person-to-person by coughing, sneezing, or close contact. People can carry the pneumococcal infection in their nose and throat without being sick and can easily spread the bacteria to others. This means that pneumococcal disease can cause complications that range from mild to very severe.
When pneumococcal bacteria spread from the nose and throat to the ears or sinuses, it typically causes mild infections, such as ear and sinus infections. However, when the bacteria spreads to other parts of the body, it can cause life-threatening illnesses like pneumonia, septicaemia and meningitis.
When pneumococcal infection leads to these more serious complications, it is known as invasive pneumococcal disease (IPD).
Anyone can get pneumococcal disease, and you can get it any time of the year. However, most cases tend to occur in the winter months. Older adults, the very young and those with a weakened immune system are most at risk of severe illness and IPD.
All those aged 65 and older are at risk of pneumococcal disease and invasive infection. Other risk factors include having diabetes; a weakened immune system due to disease or treatment; Down syndrome; or certain chronic conditions.
Over the years, the pneumococcal bacterium has become resistant to many medications. This makes treating pneumococcal disease much more difficult. But it also means that prevention of pneumococcal disease by vaccination is more important than ever. The pneumococcal polysaccharide vaccine, PPV23, offers the best way protection against pneumococcal infection and IPD.
There is no change in the recommendations for pneumococcal polysaccharide vaccine, PPV23, ie, for those aged 65 years and older and those in specific at-risk groups.
It is important to remember that patients aged 65 years and older who have never previously received PPV23 require a once only dose of PPV23.
Administering PPV23 to those we care for who are most at-risk is the best way to protect them from pneumococcal disease.
A new HSeLanD training module called Pneumococcal Polysaccharide Vaccine (PPV23) and algorithms (see Figures 3 and 4) have been developed on the PPV23 programme.
RESOURCES TO SUPPORT VACCINATORS
Public information materials about influenza vaccines, including HSE patient leaflets and posters, are all available on www.hse.ie/flu.
Information for healthcare workers is also available on www.immunisation.ie
HSeLanD training programmes for:
- Influenza vaccination — LAIV (2 modules) and QIV (1 module).
- Covid-19 vaccination.
- Pneumococcal polysaccharide vaccine PPV23. These are available at www.hseland.ie
REFERENCES
Antonova EN, Rycroft CE, Ambrose CS, Heikkinen T, Principi N. Burden of paediatric influenza in Western Europe: a systematic review. BMC Public Health. 2012 Nov 12;12:968.
NACI. Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2022/2023. Available at: https://www.canada.ca/ en/public-health/services/ publications/vaccines-immunization/ canadian-immunization-guidestatement-seasonal-influenzavaccine-2022-2023.html.
Finnish Institute for Health and Welfare. Vaccination programme for children and adolescents [online]. Available from: https://thl.fi/en/web/ vaccination/national-vaccinationprogramme/vaccinationprogrammefor-children-and-adolescents.
Health Protection Surveillance Centre (HPSC). Annual Epidemiological Report, Influenza and Other Seasonal Respiratory Viruses in Ireland, 2018/2019. (December 2019). Available at: https://www.hpsc. ie/a-z/respiratory/influenza/ seasonalinfluenza/surveillance/ influenzasurveillancereports/previo usinfluenzaseasonssurveillancerep orts/20182019season/Influenza%20 2018-2019%20Season_Summary.pdf.
HSE Seasonal Influenza Vaccination Programme 2022/2023. Available at: https://www.hse.ie/eng/health/ immunisation/hcpinfo/fluinfo/.
Caspard H, Mallory R M, Yu J, Ambrose CS. Live-Attenuated Influenza Vaccine Effectiveness in Children from 2009 to 2015–2016: A Systematic Review and MetaAnalysis. Open Forum Infect Dis. 2017 Summer; 4(3): ofx111.
National Immunisation Advisory Committee. (2022). Immunisation Guidelines Chapter 11 – Influenza (online). Available from: https:// www.hse.ie/eng/health/ immunisation/hcpinfo/guidelines/ chapter11.pdf.
National Immunisation Advisory Committee. (2022). Immunisation Guidelines Chapter 5a – COVID-19 (online). Available from: https://www. hse.ie/eng/health/immunisation/ hcpinfo/guidelines/covid19.pdf.
Quadrivalent Live Attenuated Influenza (LAIV), nasal, Fluenz Tetra (AstraZeneca Pharmaceuticals (Ireland) DAC) SmPC. Available at: https://www.ema.europa.eu/en/ medicines/human/EPAR/fluenztetra#product-information-section.
National Health Service. The NHS National Flu Immunisation Programme 2022/2023. Available at: www.gov.uk/government/ publications/national-fluimmunisation-programme-plan.
Pebody RG, Green HK, Andrews N, Zhao H, Boddington N, et al. Uptake and impact of vaccinating school age children against influenza during a season with circulation of drifted influenza A and B strains, England, 2014/15. Surveillance and Outbreak report. www.eurosurveillance.org/images/ dynamic/EE/V20N39/art21256.pdf.
World Health Organisation. Influenza Update N° 427. 2022. Available from www.who.int/ publications/m/item/influenzaupdate-n-427.