Donna Cosgrove MPSI looks at the role of the pharmacist in delivering vital vaccine services in Ireland
Pharmacy winter vaccination programme Pharmacists in Ireland have been providing influenza vaccination services (and adrenaline for emergency treatment of anaphylaxis) since 2011. These services have since expanded to include a broader range of vaccinations and emergency medicines. In 2024,
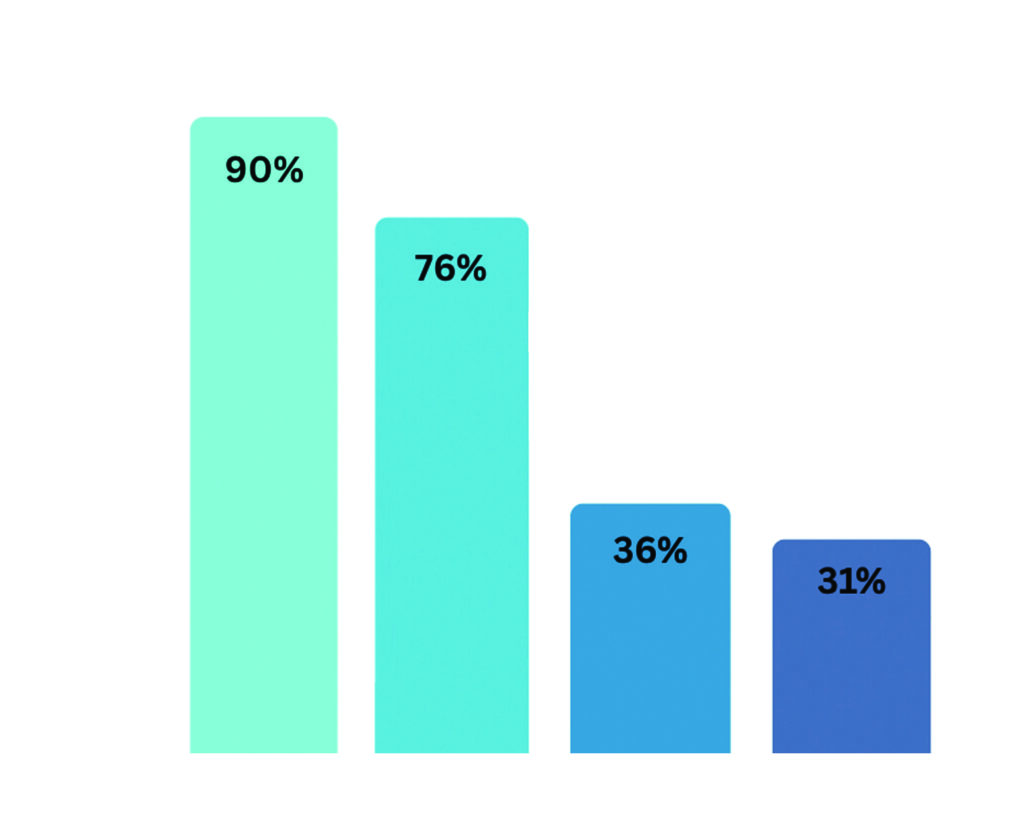
the PSI reported that 58 per cent of pharmacists were trained to provide these. Figure 1 shows a breakdown of the specific types of vaccination administration training completed by these vaccinating pharmacists.1
The HSE’s winter vaccination programme began in October, with the rollout of both influenza and Covid-19 vaccines. These can both be serious illnesses that lead to complications, hospitalisation, ICU admissions and deaths.2 Nearly 6,500 people were admitted to hospital with influenza, and over 2,000 with Covid-19, in the winter of 2024.
Vaccination is the most effective way for individuals to protect themselves from the morbidity and mortality linked to these diseases. Both vaccines are available in participating pharmacies and are free
of charge for individuals for whom it is recommended by the HSE.
If a person has one or more of the following underlying medical conditions and risk factors, the HSE recommends3 influenza and Covid-19 vaccination
- Cancer
- Chronic heart disease
- Chronic kidney disease
- Chronic liver disease
- Chronic neurological disease.
- Chronic respiratory disease.
- Diabetes and other metabolic disorders, including inherited metabolic disorders.
- Haemoglobinopathies.
- Immunocompromise due to disease or treatment.
- Body mass index ?40kg/m2.
- Serious mental health conditions.
- Children and adults with Down syndrome.
- Children with moderate-to-severe neurodevelopmental disorders (influenza vaccine only).
- Children on long-term aspirin therapy (influenza vaccine only).
Influenza and Covid-19 vaccines can be administered at the same time, ideally in different arms.
Influenza
This is an acute, self-limiting illness of the respiratory tract that also gives rise to systemic symptoms. Influenza symptoms include abrupt onset of fever, headache, myalgia, cough, sore throat, and malaise. Influenza is transmitted by aerosol (small, airborne particles that penetrate into the lungs), droplets, or contact with items recently contaminated with infected respiratory secretions.
It is generally contagious for one- to-two days before symptom onset, to four-to-five days after. Recovery usually occurs in two-to-seven days, but it can be severe. Infection may be asymptomatic
in as much as 75 per cent of cases, but outbreaks result in significant morbidity, including due to pneumonia (primary viral or secondary bacterial). Eighty-to-90 per cent of deaths from influenza occur in older people due to secondary bacterial pneumonia or exacerbations of COPD or cardiac disease.4
Influenza is a very infectious RNA virus, with types A, B and C causing human infection, most commonly types A and B. Influenza A viruses have two surface antigens, haemagglutinin (H) and neuraminidase (N) and can infect a range of animal and avian species.
Based on their H and N content, the viruses are divided into subtypes. H1, H2, H3, N1 and N2 subtypes have been implicated in widespread human infection. Minor changes (antigenic drift) occur each year between flu seasons, which is the reason for changing the vaccine composition each year. Vaccines each year are prepared based on recommendations from the World Health Organisation, using virus strains that are most likely to circulate. Major changes (antigenic shift) occur occasionally, creating an immunologically distinct virus with the potential for high morbidity and mortality.
Influenza B viruses generally result in a less severe illness than influenza A, and only infect a limited host range by comparison, ie, humans and seals. This is probably the reason that influenza B has not caused any pandemics.
They are divided into two lineages, B/ Victoria and B/Yamagata. WHO no longer recommends inclusion of a B/Yamagata antigen component because these lineages do not appear to be circulating any more. Trivalent (as opposed to quadrivalent, protecting against four strains) vaccines are recommended for the current flu season. Influenza C commonly causes mild upper respiratory tract illness, infecting humans and pigs. Most individuals have acquired immunity by adulthood.
| GROUPS | INFLUENZA VACCINE | COVID-19 VACCINE |
| ?60 years (this is recommended by the HSE, although NIAC recommends ?60 years) | ||
| 2-to-17 years | Those with immunocompromise or an underlying medical condition | |
| 18 years and older living in long- term care facilities for older adults | ||
| 6 months and older with immunocompromise | ||
| 6 months and older with an underlying medical risk factor | ||
| Pregnancy | If in another risk group; Available in each pregnancy for those who choose to receive a vaccine | |
| Healthcare workers (HCWs) | If in another risk group; Available in each pregnancy for those who choose to receive a vaccine | |
| Carers and household contacts of people with underlying chronic health conditions | Not unless in another risk group | |
| People with close, regular contact with pigs, poultry or water fowl | Not unless in another risk group | |
| Adults aged 18-59 years without underlying medical risk factors | Not recommended | One dose should be available each year for those who choose to receive a vaccine following discussion with a healthcare provider |
Table 1: Summary of groups recommended to receive influenza and Covid-19 vaccines*3
*This list is not exhaustive, and the medical practitioner should apply clinical judgment to consider the risk of influenza exacerbating any medical condition that a patient may have, as well as the risk of serious illness from influenza
Post-vaccination, influenza antibodies take about 10-to-14 days to reach protective levels, offering a protection level of 40-to- 60 per cent, which lasts for about a year. All influenza vaccines are contraindicated in people who have had anaphylaxis after a previous dose of influenza vaccine or its constituents, and in those with severe neutropaenia in order to avoid an acute vaccine related febrile illness.
LAIV is contraindicated in children aged two-to-17 years:
- With severe immunocompromise due to disease or treatment.
- Living with severely immunocompromised persons.
- Experiencing an acute exacerbation of asthma, including those who have had increased wheezing or needed additional bronchodilator treatment in the previous 72 hours .
- Taking systemic aspirin or other salicylates.
- Who have taken influenza antiviral medication within the previous 48 hours.
- Who are pregnant.
All influenza vaccines are contraindicated in people with:
- Acute severe febrile illness.
- Egg allergy: Those with confirmed egg anaphylaxis or egg allergy can be given influenza vaccine in a primary care or school setting, with the exception of those who have required admission to ICU for a previous severe anaphylaxis to egg.
- Receiving combination checkpoint inhibitor therapy such as ipilimumab plus nivolumab.
Three types of influenza vaccine are available this 2025/2026 season are:5
- A live attenuated influenza vaccine (LAIV)-trivalent, containing antigens from two type A and one type B virus strain (cultured in hens’ eggs or cell lines). This vaccine is provided as a nasal spray for those aged two-to-17 years, called Fluenz.
- An inactivated influenza vaccine (IIV)-trivalent, containing antigens from two type A and one type B virus strain (cultured in fertilised hens’ eggs or cell lines). This is supplied in a prefilled syringe for intramuscular administration. There are two available brands, Influvac and Vaxigrip.
Covid-19
There are seven coronaviruses that can cause disease in humans. Four of these only cause minor respiratory illnesses, whereas the other three (MERS-CoV, SARS-CoV, and SARS CoV-2, also known as Covid-19) cause more severe disease.6 Mutations can occur, as with most RNA viruses, and multiple variant strains of Covid -19 have been identified. The growth potential and mutation profile of variants is monitored in case this might impact the effectiveness of the vaccines.
In March 2020, the World Health Organisation declared an outbreak of Covid-19 as a pandemic. The severity of Covid-19 infection reduced over time due to vaccination and natural infection establishing immunity in the population, and evolution of the virus. Older adults, infants, the immunocompromised, and those with underlying medical conditions have the highest risk of hospitalisation from Covid -19. Transmission occurs mainly between people who have been indoors and within two metres for a total of 15 minutes over a 24-hour period, and the incubation period is usually about two-to-five days. Covid-19 is infectious from two days before symptom onset, peaking within five days of symptom onset. Common symptoms include cough, fatigue, sore throat, fever, headache, hoarse voice, and sneezing. The Covid-19 vaccine is safe, with the benefits strongly outweighing any risk – more than 5.5 million people have so far received it. The EMA has licensed multiple types of vaccines for Covid-19 – viral vector vaccines, protein subunit vaccines, and messenger RNA vaccines. Initial clinical trials of Covid-19 vaccines in previously unvaccinated people reported an efficacy of 85-to-100 per cent. However, booster vaccinations were required due to the emergence of variants and the protection gradually waning over time; although protection against more severe disease is more durable. Severe illness and death is more likely in individuals aged 65 and older, with one or more of the medical conditions listed above, and those from Black, Asian and minority backgrounds. Due to the evolution and emergence of different Covid-19 variants, now pregnant women do not have any increased risk of developing Covid-19 compared to non-pregnant women. Covid-19 vaccines are effective in preventing hospitalisations, severe disease, and death secondary to Covid-19. Protection against mild infection is limited, however, and it is more durable against severe disease.
Vaccine effectiveness varies, depending on the variant targeted by the vaccine and the strain of the virus causing the infection, but estimates range from 41-to-57 per cent effectiveness against Covid-19 infection, and 64-to-76 per cent against hospitalisation. Following vaccination, peak protection is established at four- to-eight weeks and wanes thereafter. Hybrid immunity (the combination of immune protection from both infection and vaccination) offers a stronger, more durable protection than either alone, and can persist for up to 12 months. This is not the case in older age groups or immunocompromised individuals, however. The aim of the Covid-19 vaccination programme is to offer year-round protection against severe disease in at-risk populations. To achieve this, one vaccination per year is sufficient for many at-risk people, but in certain populations NIAC recommends twice- yearly vaccinations.
The Covid-19 vaccination currently available in Ireland is Comirnaty LP.8.1 (mRNA based), with different strengths indicated depending on the age group. This is available as a multidose vial, for intramuscular administration. Anaphylaxis after an mRNA vaccine or after polyethylene glycol administration are contraindications to receiving the vaccine. Unfortunately, no alternative non-mRNA vaccine is available here. Those interested in receiving a protein subunit vaccine (ie, Novavax) can contact HSELive for updates.
More detailed information on winter vaccines is available from the HSE website.
References
1. Pharmaceutical Society of Ireland. (2025). Survey of the Register Report 2024. Available https://www.psi.ie/sites/default/files/document/PSI_Survey_of_the_Register_Report_2024.pdf.
2. Health Service Executive. (2025). HSE’s Winter Vaccination Programme starts today [Press release]. Available https://about. hse.ie/news/hses-winter-vacci- nation-programme-starts-today/ [Accessed 19 Oct 2025].
3. Health Service Executive. (2025). Summary of groups recommended to receive influ- enza and COVID-19 vaccines in autumn/winter 2025/2026. Available https://www.hse.ie/eng/health/immunisation/hcpin-fo/fluinfo/recommendedgroupscovid19andinfluenza.pdf [Accessed 19 Oct 2025].
4. National Immunisation Adviso- ry Committee. (2025). Immu- nisation Guidelines for Ireland: Chapter 11 Influenza. Available https://www.hiqa.ie/sites/default/ files/NIAC/Immunisation_Guide- lines/Chapter_11_Influenza.pdf [Accessed 19 Oct 2025].
5. Health Service Executive. (2025). Immunisation – Health- care Worker Information. Avail- able https://www.hse.ie/eng/ health/immunisation/hcpinfo/ fluinfo/ [Accessed 19 Oct 2025].
6. National Immunisation Adviso- ry Committee. (2025). Immu- nisation Guidelines for Ireland: Chapter 5a COVID-19. https:// www.hiqa.ie/sites/default/files/ NIAC/Immunisation_Guidelines/ Chapter_05a_COVID-19.pdf [Accessed 19 Oct 2025].