Damien O’Brien examines the different levels of pain and the variety of pharmacological and non pharmacological treatments
Pain is an unpleasant sensory and emotional experience associated with potential or actual damage to tissue. It is subjective and individual to each person1. Pain can be classified based on the duration of pain – with pain of a duration of less than three months defined as acute pain and pain of a duration of more than three months defined as chronic pain2. Additionally, the World Health Organization (WHO) also classifies pain based on the severity – mild, moderate or severe3.
Pain is one of the main symptoms of many illnesses, with it being estimated as the chief complaint for 52% of emergency department visits4. Acute pain can be useful as it warns against a dangerous situation and causes an individual to withdraw from the situation. Chronic pain can be very debilitating and reduce a patient’s quality of life2.
Mechanism of pain
There are three main mechanisms of pain – nociceptive, neuropathic and psychogenic. Nociceptive pain is caused by stimulation of sensory nerve fibres to a harmful intensity. Nociceptive pain can be classified based on the mode of stimulation – with thermal (hot or cold), mechanical (tearing or crushing) and chemical (chemicals released during inflammation) the most common modes.
Nociceptive pain can also be classified according to origin site and divided into superficial somatic, deep somatic and visceral pain. Superficial somatic pain is caused by stimulation of nociceptors in the skin or other superficial tissue. It tends to be sharp and clearly located. Deep somatic pain is caused by activation of nociceptors in bones, ligaments, tendons, blood vessels and muscles. Deep somatic pain is dull, aching and poorly localised. Visceral pain can be felt widespread and is difficult to locate.56.
Neuropathic pain is caused by damage to either the central or peripheral nervous systems. This can be a mechanical injury or can be from other factors that affect the nervous system and includes multiple sclerosis, chemotherapy or alcoholism. Psychogenic pain is caused, prolonged or increased by mental or emotional factors56.
WHO analgesic ladder
The WHO analgesic ladder was introduced in 1986 to provide guidance for pain relief in cancer and palliative care patients. It has been modified many times over the years and is now used to treat both acute and chronic pain caused by a wide array of diseases and not only cancer. The updated analgesic ladder is shown below and now contains a fourth step7.
The main principles of the analgesic ladder are “by the clock, by the mouth, by the ladder”. This means that analgesics should be:
Taken at regular intervals. This allows the pharmacokinetic characteristics of the drug to be followed. The half-life, duration of action and bioavailability should all be considered and allows analgesics for chronic pain to be taken on a regular basis and not on an ‘as required’ basis.
Taken orally, when possible, as opposed to intravenous, rectal etc. The oral route provides a much simpler route of administration.
Prescribed at step 1 and titrated upward as required7.
Analgesics should be prescribed according to the pain intensity, with each patient properly undergoing a clinical examination. Individualised treatment is important as dosage may have to be adjusted according to patient response, balancing desired effects with adverse effects. Medication compliance is important as medication omissions can lead to reoccurrence of pain7. The bidirectional approach in the updated ladder is useful for both acute and chronic pain. For chronic pain, a stepwise approach titrating up from the bottom to top is often used. For acute pain, a stronger analgesic for the intensity of the initial pain is often used and then gradually titrated downwards7.
One of the main limitations of the original ladder is that it didn’t allow the integration of non-pharmacological treatments into the patient’s treatment plan. Another limitation of the ladder is that it places NSAIDs at the bottom of the ladder and opioids on step two, which can lead to a false belief that they represent a safe treatment – which is not always the case. The analgesic ladder also doesn’t consider the management of neuropathic pain, which has a complex pathophysiology and requires a different treatment plan3.
Treatment
It is important to treat pain due to the fact that patients that are suffering from chronic pain can have their quality of life greatly reduced. This tends to depend on the intensity and duration of the pain, and not the specific cause of the pain. Common disorders involving sleep, appetite, libido and fatigue are often observed in chronic pain patients. Additionally, patients with chronic pain often feel anxious, depressed and irritated8. Appropriate treatment of pain necessitates an understanding of the pain characteristics, including the severity and nature of the pain9. There are a wide range of pharmacological and non-pharmacological treatment options available to relieve pain outlined below.
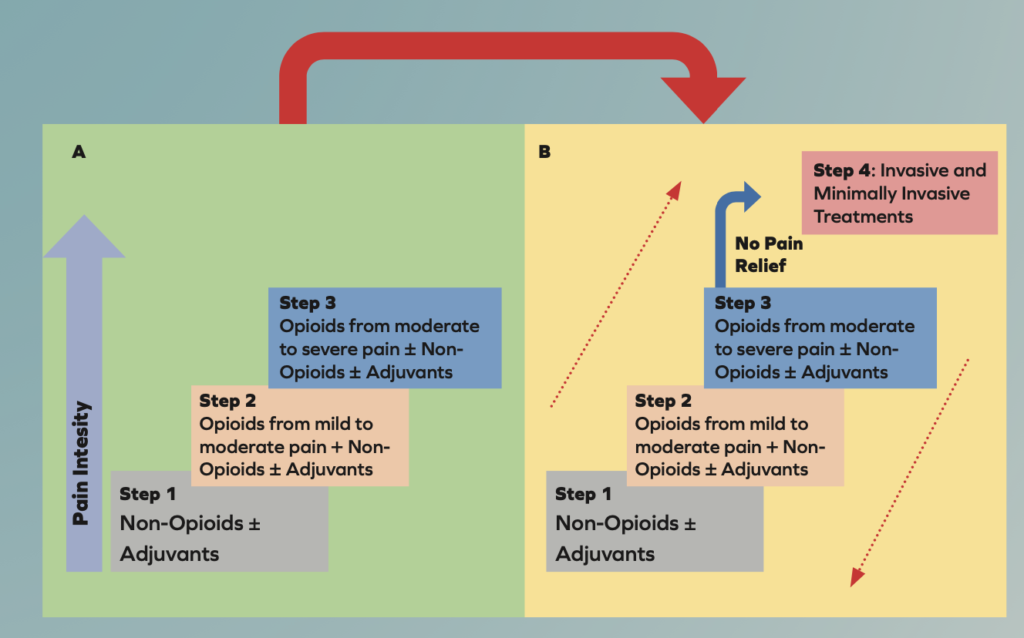
Transition from the original WHO three-step analgesic ladder (A) to the revised WHO fourth-step form (B). The additional step 4 is an “interventional” step and includes invasive and minimally invasive techniques. This updated WHO ladder provides a bidirectional approach.
Pharmacological treatment
Paracetamol
The mechanism of action for paracetamol is not fully clear. Unlike non-steroidal anti-inflammatory drugs (NSAIDs), paracetamol does not inhibit cyclooxygenase (COX) enzymes 1 or 2. It is hypothesised that it may inhibit a different variant known as COX-3, but it is still unclear. It does not have anti-inflammatory properties, but it does have analgesic and antipyretic properties. Paracetamol is effective in treating mild-to-moderate nociceptive pain but there is no evidence that it is effective in treating neuropathic pain. Paracetamol is a safe drug when used correctly, with a favourable adverse effect profile10. The maximum recommended dose of paracetamol for adults (aged 12 years and older) is 1g every 4-6 hours, up to 4g per day. In children, a weight-based dosing approach is more appropriate – with 15mg/kg per dose recommended. Paracetamol overdose may cause liver damage or failure. It can be administered orally, rectally or intravenously11,12.
NSAIDs
The main mechanism of action of NSAIDs is the inhibition of COX enzymes, both COX-1 and COX-2. This inhibits the synthesis of prostaglandins, which are mediators involved in inflammation and pain. NSAIDs are effective in treating mild-to-moderate pain, reducing inflammation and reducing fever. Similarly to paracetamol, there is no evidence that NSAIDs are effective in treating neuropathic pain but they are effective in treating nociceptive pain. NSAIDs can be classified based on their specificity for COX-1 or COX-210.
COX-1 enzymes are found in many organs and play an important role in platelet aggregation and gastrointestinal (GI) protection, while COX-2 enzymes are mainly involved in the inflammatory response. Nonselective NSAIDs include ibuprofen, diclofenac and naproxen inhibit both COX-1 and COX-2 enzymes. Due to their COX-1 inhibition, these nonselective NSAIDs are more likely to cause GI adverse effects. Selective COX-2 inhibitors have a significantly more favourable GI adverse effect profile but may be more expensive13.
Several NSAIDs are licensed for use in Ireland. The choice of the drug depends on a variety of factors including co-morbidities, bleeding risk, cost, availability, dosing schedule and adverse effect profile. The response to different NSAIDs can vary widely between patients and the mechanism for the partial response is not fully understood1013. Ibuprofen and aspirin are available OTC in Ireland, while celecoxib, etoricoxib, diclofenac, meloxicam, naproxen, dexketoprofen, aceclofenac and mefenamic acid are among the NSAIDs available on prescription. Celecoxib and etoricoxib have the greatest selectivity for COX-2 and therefore has the best GI adverse effect profile. However, these may be associated with an increased risk in cardiovascular events. If a patient has had previous cardiovascular problems, a COX-2 inhibitor may be best to avoid. A COX-2 inhibitor may be more suitable if the patient has had GI problems in the past. A thorough patient history should be taken, and an individualised plan should be followed for each patient. Topical NSAIDs can also be beneficial while avoiding many systemic adverse effects13.
Healthy patients that are only taking NSAIDs in the short term may not need regular monitoring. However, patients that are a higher risk and taking NSAIDs for long-term use may require regular full blood test, renal tests and hepatic tests14. The cautions for use and contraindications are similar for the different NSAISDs and some of them are outlined below:
Increasing age
Presence of a peptic ulcer
History of GI bleeds
Renal disease
Inflammatory bowel disease
Pregnancy
Breastfeeding
Myocardial infarction
Transient ischemic attack
Congestive heart failure
Stroke10.
Due to the risk of adverse effects, the lowest effective dose should be used for the shortest time possible. A proton pump inhibitor can be used to mitigate the GI risk of nonselective NSAIDs; however, this can contribute to polypharmacy and increased cost13. Several adverse effects related to different body system are possible and the most common are outlined below:
Gastrointestinal – nausea, anorexia, dyspepsia, abdominal pain, ulcers, GI haemorrhage, constipation, diarrhoea
Cardiovascular – hypertension, myocardial infarction, stroke, thromboembolic events
Renal – salt and water retention, reduced kidney function, oedema, hyperkalaemia
Central nervous system – headache, dizziness, vertigo, confusion, lowered seizure threshold
Hepatic – hepatotoxicity
Hypersensitivity – rhinitis, asthma, urticaria, flushing10,15.
Opioids
Opioids exert their mechanism of action by binding and activating mu, kappa and delta opioid receptors in the nervous system. Presynaptically, opioids block calcium channels on nociceptive nerves and inhibits the release of neurotransmitters including glutamate and substance P. Postsynaptically, opioids enhance potassium channel activity, which hyperpolarises cell membranes and increases the action potential required to generate nociceptive transmission10.
As per the WHO analgesic ladder, the oral route is the preferred route of administration but there are a number of different routes including transdermal, intramuscular, intravenous, epidural and transmucosal. Opioids are recognised as the most effective drugs in treating severe pain. Opioids should only be used if the expected benefit for both pain and function outweighs the risk of addiction, tolerance, and adverse effects. Opioids should be used at the lowest effective dose and for the shortest duration10.
As per the older version WHO analgesic ladder, weaker opioids such as codeine and dihydrocodeine were used on step two of the ladder. The term weak opioid should not encourage a lack of caution in use. Strong opioids have a much broader dose range and a proportionately greater effect can be achieved by a dose increase. The definition of an opioid being strong or weak reflects the pharmacodynamic (potency and efficacy) of an opioid more so than the pharmacokinetic properties. In addition to being less effective, there is evidence that weak opioids may be more addictive than strong opioids. Buprenorphine, morphine, hydromorphone, methadone, oxycodone and fentanyl are examples of strong opioids licensed for use in Ireland16,17. In the new WHO guidance issued in 2018, there is no pharmacological rationale for the use of weak opioids. The new guidance recommends the use of lower doses of strong opioids, with or without a non-opioid agent, and with or without adjuvant17.
Tramadol is an interesting case as it is classified by the WHO as a weak opioid and by the British National Formulary as a strong opioid. Tramadol is a prodrug and acts as an agonist at the mu opioid receptor. Due to variable CYP2D6 metabolism, there is wide individual variation in both adverse effect and analgesic effect – reduced analgesic effect for poor metabolisers and increased adverse effects in rapid metabolisers. It is an atypical analgesic as it also has serotonin/norepinephrine reuptake inhibitor (SNRI) effects. The SNRI mechanism contributes to an increased risk of serotonin syndrome and drug-drug interactions17.
The contraindications and cautions in use can vary depending on the opioid agent, but some are outlined below:
Severe respiratory instability
Acute mental instability
History of substance abuse
Intolerance or serious adverse effects to other opioids
Renal impairment
Hepatic impairment10.
There are several adverse effects associated with opioids and these are outlined below. The adverse effects are similar across the different opioids, but differences may be observed due to interpatient variability. Opioid-induced constipation is extremely common and therefore laxatives should be co-administered to prevent constipation. An osmotic laxative like lactulose or stimulant laxative like senna or bisacodyl can be co-administered to prevent constipation10,18.
Sedation
Constipation
Nausea and vomiting
Respiratory depression
Euphoria or dysphoria
Bradycardia
Hyperalgesia or allodynia
Tolerance
Physical dependence10.
Patients should be monitored regularly for level of pain control, hepatic function, renal function and a physical examination including vital signs, signs of misuse and respiratory status. Opioid overdose can cause severe respiratory depression and ultimately lead to death. Respiratory depression due to opioid toxicity can be treated with IV, IM or intranasal naloxone10.
Opioids should be used at the lowest effective
dose and for the shortest duration
Antidepressants
Both tricyclic antidepressants (TCAs) and SNRIs inhibit the reuptake of two neurotransmitters, serotonin and noradrenaline, which increases the descending inhibitory pathways of the nervous system related to pain. SNRIs, particularly duloxetine, and TCAs, especially amitriptyline, have shown clear efficacy in treating neuropathic pain and are recommended as first line treatment. Additionally, they can be used for other conditions including fibromyalgia, chronic musculoskeletal pain and amitriptyline is often used prophylactically in treating migraine and headaches. Both groups are more effective in treating patients that have depressive symptoms as a comorbidity. The most common adverse effects include nausea, vomiting, decreased libido, drowsiness, dry mouth, headache and weight gain. Contraindications and cautions in use include coadministration with or within 14 days of monoamine oxidase inhibitors (MAOIs), hepatic impairment, renal impairment and cardiac disease. Patients should be initiated for six to eight weeks for an adequate trial. If pain relief is achieved but adverse effects present, the patient may be switched to another TCA or SNRI. The patient should be monitored for signs of serotonin syndrome, particularly if the patient is on other drugs that may increase serotonin in the CNS10.
Antiepileptic
Voltage-dependent calcium channels are overexpressed in patients with neuropathic pain. Pregabalin and gabapentin both reduce calcium-dependent release of excitatory neurotransmitters and therefore decrease neuronal excitability and reduce neuropathic pain. Gabapentin and pregabalin are commonly used to treat neuropathic pain, while oxcarbazepine and carbamazepine are often used to treat trigeminal neuralgia. The dose of gabapentin and pregabalin should be started at a low dose and titrated upwards. Adverse effects of antiepileptics are similar and include dizziness, blurred vision, nausea, headache, confusion and depression. The main caution in use is renal impairment – with dose adjustment necessary for patients with reduced renal function. Baseline creatinine levels should be evaluated before and during treatment. Patients should also be screened for behavioural changes10.
Non-pharmacological treatment
Several non-pharmacological treatments can be effective in relieving pain and the WHO ladder has been updated to reflect this. These treatment options can be used alone or combined with other pharmacological or non-pharmacological treatments to form a multicomponent approach. Non-pharmacological options are often relatively inexpensive and have a favourable adverse effect profile. They can reduce the anxiety associated with pain and reduce the dosage of analgesic drugs needed, therefore reducing the adverse effects of these drugs19.
Physiotherapy and exercise are very important in the non-pharmacological management of pain. Exercise has been shown to have effectiveness in reducing pain in patients. Exercise prescription from a trained and component physiotherapist can be a very useful intervention in chronic pain patients and should address any biomechanical and psychosocial issues that contribute towards the pain. Patient education, a multidisciplinary approach and a correct exercise plan that is safe and effective is important in a successful rehabilitation programme20,21.
Acupuncture and Tai Chi are other non-pharmacological interventions that can provide pain relief for patients. These methods generally are safe when performed by an appropriately trained professional. However, there is a risk of injury with Tai Chi when improper instructions are used, as well as a risk of infection at the site of administration with acupuncture22. Cognitive behavioural therapy (CBT) has been shown to be effective in treating patients with chronic pain. It can be used as a standalone treatment or as an adjunct to other therapies23.
References on request