Donna Cosgrove MPSI explores hyperglycaemia, insulin resistance, examples of drugs that may impair glucose tolerance, and treatments — both current and future
Type 2 diabetes is characterised by hyperglycaemia, insulin resistance and impairment in insulin secretion.1 Diagnosis is made based on measures that show evidence of elevated glycaemia due to both issues with insulin secretion (beta cell dysfunction) and insulin action, or resistance. Resistance to insulin may contribute to further physiological abnormalities in type 2 diabetes such as inflammation, lipoprotein abnormalities, hypertension, and further metabolic issues. Metabolic syndrome refers to type 2 diabetes when accompanied by additional clinical conditions like hypertension, dyslipidemia, and central obesity; issues that may be caused at least in part due to insulin resistance.
Pathophysiology of type 2 diabetes
The cause of insulin resistance is commonly attributed to environmental factors related to increased food intake and a sedentary lifestyle, which causes overweight and obesity. Genetics and ageing also contribute. Issues with insulin secretion result from genetics and how pancreatic beta cells are made and programmed in utero. The presence of hyperglycaemia can itself also impair beta cell function, further exacerbating the problem.1
Studies have shown that defects in beta cell function can occur early in disease pathogenesis, even before the development of obesity and insulin resistance. Substances released by adipocytes (adipokines, eg leptin, adiponectin, TNF alpha, resistin) may, at least in part, cause insulin resistance. Insulin release involves the cleavage of proinsulin to insulin. In type 2 diabetes, there is an increase in proinsulin secretion, suggesting that this processing is somehow impaired in the beta cells in type 2 diabetes. Insulin resistance becomes more pronounced with increasing age and weight, revealing any underlying defect in beta cell function in affected individuals. The mechanism through which obesity causes insulin resistance, however, is poorly understood. Some studies have investigated the role of inflammation in the development of type 2 diabetes (and also atherosclerosis), the incidence of which is correlated with increased levels of inflammatory markers such as C reactive protein, IL6, TNF alpha, chemokines and adipokines. These inflammatory markers can be reduced by intensive lifestyle interventions. Interestingly, the anti-inflammatory effects of medications like statins may provide a greater therapeutic benefit beyond their intended effect. Use of anti-inflammatory medications even in other diseases such as rheumatoid arthritis and psoriasis is linked with a lower incidence of type 2 diabetes.
Drug induced hyperglycaemia
Certain drugs impair glucose tolerance through reducing insulin secretion, increasing hepatic glucose production, or causing insulin resistance (Table 1). Where appropriate, patients should be informed about these risks (especially with prolonged steroid use), as with all clinically relevant side effects.
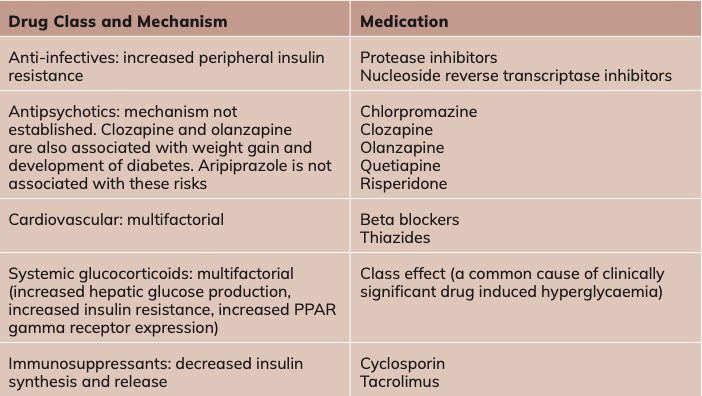
Table 1. Examples of drugs that may impair glucose tolerance
Treatment
Lifestyle interventions are a mainstay of weight management, but used alone, are associated only with moderate weight loss.2 Maintenance of weight loss is intrinsically difficult due to counter-regulatory neuroendocrine pathways that increase hunger and reduce satiety, promoting weight gain and possibly reducing energy expenditure.
- Metformin, the most common antidiabetic drug, decreases hepatic glucose production and intestinal glucose absorption, and increases peripheral glucose uptake.3 It does not affect insulin secretion;
- Dipeptidyl peptidase (DPP) 4 Inhibitors (eg, sitagliptin, saxagliptin, linagliptin) inhibit DPP-4, which deactivates GLP-1 and also the glucose dependent insulinotropic polypeptide.3 These are oral GLP-1 based therapies that increase levels of GLP-1, but are not as effective as GLP-1 agonists at glucose or weight reduction;
- Thiazolidinediones (eg, pioglitazone) increase insulin sensitivity by acting on adipose tissue and muscle to increase glucose use. They also reduce glucose production by the liver. Their mechanism of action is not fully understood, although they bind to and activate peroxisome proliferator-activated receptors (PPARs), mostly PPAR-gamma, which alters the transcription of multiple genes involved in glucose and lipid metabolism.4 Use of thiazolidinediones has decreased in recent years because of concerns about safety and side effects such as heart failure and increased risk of fractures;5
- Sulfonylureas (eg gliclazide) only benefit patients with some residual beta cell function. These drugs bind to specific receptors here, with the ultimate effect of insulin release and stimulation of new insulin granules;3
- Sodium glucose co-transporter (SGLT) 2 inhibitors inhibit SGLT2 receptors in the kidneys’ proximal convoluted tubule, resulting in the prevention of glucose reabsorption. Glucose is then excreted in the urine, helping with weight loss, but also increasing UTI risk;3
- Glucagon-like peptide (GLP) 1 agonists (eg, exenatide, liraglutide, dulaglutide, semaglutide) are injectable medications that enhance the effect of GLP-1. GLP-1 is a gastrointestinal peptide contributing to the regulation of glucose levels when released after food consumption, when it stimulates insulin formation and release. It also slows gastric emptying, inhibits excess postprandial glucagon release.3 These drugs promote weight loss by reducing energy intake, increasing satiety, reducing hunger, and improving glycaemic control. Semaglutide is a long-acting GLP-1 analogue.
Type 2 diabetes is associated with an increased risk of cardiovascular and kidney disease, and corresponding impaired quality of life and increased mortality
Type 2 diabetes is associated with an increased risk of cardiovascular and kidney disease, and corresponding impaired quality of life and increased mortality. In recent years, it has been suggested that cardiovascular and kidney disease markers in type 2 diabetes are at least, if not more, clinically important for long-term outcomes than measures of glycaemia. Two classes of antidiabetic drugs in particular have been shown to help mitigate these risks: sodium glucose cotransporter-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists.6
Future care
Two new drugs are now available to treat type 2 diabetes: finerenone (a non-steroidal mineralocorticoid receptor antagonist), and tirzepatide (a dual glucose dependent insulinotropic polypeptide (GIP)/GLP-1 receptor agonist). The National Centre for Pharmacoeconomics (NCPE) in Ireland recently recommended that the HSE should consider funding finerenone if its cost effectiveness can be improved7, while a full health technology assessment was recommended by the NCPE for tirzepatide8 to assess the clinical and cost effectiveness compared with the current standard of care.
In trials investigating finerenone, findings suggested cardiovascular and kidney benefits in both patients with type 2 diabetes and those with chronic kidney disease. Tirzepatide is also beneficial in terms of effects on weight loss and quality of life. Tirzepatide, the only drug in its class, improves quality of life and reduces body weight more significantly than any other drug. GLP-1 inhibitors are also successful in reducing body weight, with semaglutide the most effective in this class. A recent network meta analysis investigating the benefits and harms of available drug treatments for type 2 diabetes6 found that SGLT-2 inhibitors, GLP-1 receptor agonists, and finerenone showed the most benefits in terms of, eg, overall mortality reduction, fewer hospital admissions, and reducing end stage kidney disease. Predictably, the absolute benefits of these drugs are dependent on individual baseline risk levels. The authors recommend a risk stratified approach for future treatment recommendations, tailored depending on each patient’s risk profile.
Prediabetes
In prediabetes, glycaemic values are in between normal and those that define diabetes. Like type 2 diabetes, it is characterised by decreased insulin sensitivity and impaired insulin secretion. Measures of impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) based on WHO criteria can be used to diagnose the condition, although there are alternative diagnostic definitions depending on the guidelines used.9 Prediabetes identifies people at risk of progression to diabetes, but the estimated risks vary depending on the definition used (isolated IFG, or isolated IGT, a combined measure of these, or HbA1c). Intensive lifestyle intervention (ie, dietary modification and increased physical activity) and pharmacological intervention (ie, metformin) can reduce progression to diabetes. In the Diabetes Prevention Program (a large trial in the United States that compared metformin, placebo, and lifestyle interventions), intensive lifestyle intervention was found to be more effective than metformin.10
Multiple clinical guidelines, including those from NICE, stress lifestyle intervention as the preferred initial step to reduce risk of diabetes, with metformin recommended for use in certain circumstances,11 eg, if the individual’s participation in intensive lifestyle change programmes has not worked, or they are unable to participate. Unfortunately, not all individuals have access to the type of lifestyle intervention programme that proved so effective in improving glycaemic values in research studies. Orlistat may also be offered to help with obesity management where appropriate in order to improve glycaemic parameters.
References
Roberston, R.P. & Udler , M.S. (2023). Pathogenesis of Type 2 Diabetes. UpToDate. Retrieved February 24, 2024, from https://www.uptodate.com/contents/pathogenesis-of-type-2-diabetes-mellitus?search=type%202%20diabetes&source=search_result&selectedTitle=3%7E150&usage_type=default&display_rank=3
Kushner, R. F., Calanna, S., Davies, M., Dicker, D., Garvey, W. T., Goldman, B., … & Rubino, D. (2020). Semaglutide 2.4 mg for the treatment of obesity: key elements of the STEP trials 1 to 5. Obesity, 28(6), 1050-1061.
Rehani, P. R., Iftikhar, H., Nakajima, M., Tanaka, T., Jabbar, Z., & Rehani, R. N. (2019). Safety and mode of action of diabetes medications in comparison with 5-aminolevulinic acid (5-ALA). Journal of Diabetes Research, 2019.
Inzucchi, S.E. & Lupsa, B. (2022). Thiazolidinediones in the treatment of type 2 diabetes mellitus. UpToDate. Retrieved 24 February 2024 from https://www.uptodate.com/contents/thiazolidinediones-in-the-treatment-of-type-2-diabetes-mellitus?search=pioglitazone&source=search_result&selectedTitle=1%7E43&usage_type=default&display_rank=1
Lebovitz, H. E. (2019). Thiazolidinediones: the forgotten diabetes medications. Current Diabetes Reports, 19, 1-13. Available https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6881429/
Shi, Q., Nong, K., Vandvik, P. O., Guyatt, G. H., Schnell, O., Rydén, L., … & Li, S. (2023). Benefits and harms of drug treatment for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ, 381.
National Centre for Pharmacoeconomics. (2023). Finerenone (Kerendia®). HTA ID: 22012. Available https://www.ncpe.ie/finerenone-kerendia-hta-id-22012/
National Centre for Pharmacoeconomics. (2024). Tirzepatide (Mounjaro®). HTA ID: 24003. Available https://www.ncpe.ie/tirzepatide-mounjaro-hta-id-24003/
Rooney, M. R., Fang, M., Ogurtsova, K., Ozkan, B., Echouffo-Tcheugui, J. B., Boyko, E. J., … & Selvin, E. (2023). Global prevalence of prediabetes. Diabetes Care, 46(7), 1388-1394.
Diabetes Prevention Program Research Group. (2002). Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine, 346(6), 393-403.
Hostalek, U., & Campbell, I. (2021). Metformin for diabetes prevention: update of the evidence base. Current Medical Research and Opinion, 37(10), 1705-1717.
Author: Dr Donna Cosgrove PhD. Donna’s overall aim is to improve patient outcomes through education. After graduating with a BSc in Pharmacy, she returned to university to complete a MSc in Neuropharmacology.
This led to a PhD investigating the genetics of schizophrenia, followed by a postdoctoral research position in the same area. She has worked in hospital, research and community pharmacy settings and is currently a community pharmacist in Galway and a clinical writer.