Donna Cosgrove MPSI looks at reproductive health, including the main causes of infertility and the various treatment processes
Conception and infertility Fertility is the capacity to establish a clinical pregnancy, whereas infertility is when a clinical pregnancy has not been established after 12 months of regular, unprotected sexual intercourse. Conception (or fertilisation) is when sperm and an egg join together. Up to 12 per cent of couples of reproductive age — more than 186 million people — are affected globally.¹ The most predictive factor for fertility is a woman’s age, but there are many other factors at play, including² alcohol intake, smoking, obesity, low body weight, tight underwear for men (contributing to elevated scrotal temperature), and drug use (certain prescribed, recreational, or OTC medications). Investigations into the cause of infertility are recommended when a couple have been trying to conceive (TTC) for 12 months when the woman is under 35 years old, or after six months of TTC when the woman is aged over 35 years, or when there is a known condition that may affect fertility for either member of the couple.³ The main causes of infertility in the UK are:²
- Unexplained (no identified male or female cause; 25 per cent).
- Ovulatory disorders (25 per cent).
- Tubal damage (20 per cent).
- Factors in the male causing infertility (30 per cent).
- Uterine or peritoneal disorders (10 per cent). In about 40 per cent of cases, disorders are found in both the man and the woman.
Trends in fertility
In Ireland, the number of births has fallen from 67,462 in 2014, to 54,062 in 2024.4 The average age at which a woman becomes a mother has increased to 31.7 years, compared to 28.5 years in 2004. The fertility rate, or estimated number of births a woman will have in her lifetime, has also fallen from two to 1.5. Generally, a value of 2.1 is considered to be the number at which the population would replace itself in the long run. Projecting the likely fertility rates decades into the future is open to significant uncertainty, however, the UN predictions5 show only a 10 per cent probability that the global total fertility rate will exceed 2.1 by the year 2100 (Figure 1). The most recent estimate (2.2 births per woman in 2014) is lower than the original prediction made in 2013 (2.4 births per woman). Fertility transition is a concept that refers to the transition from a high to a low fertility level in a population over time. The factors that contribute to fertility transitions are diverse, but generally speaking, as economies develop in society, the socioeconomic benefits that were previously derived from having large families are no longer as significant as they were previously. Declining infant mortality rates result in more children surviving, and the cost of raising a child has increased as parents invest more in their development, ie, education, nutrition, healthcare.
Assisted reproductive technology
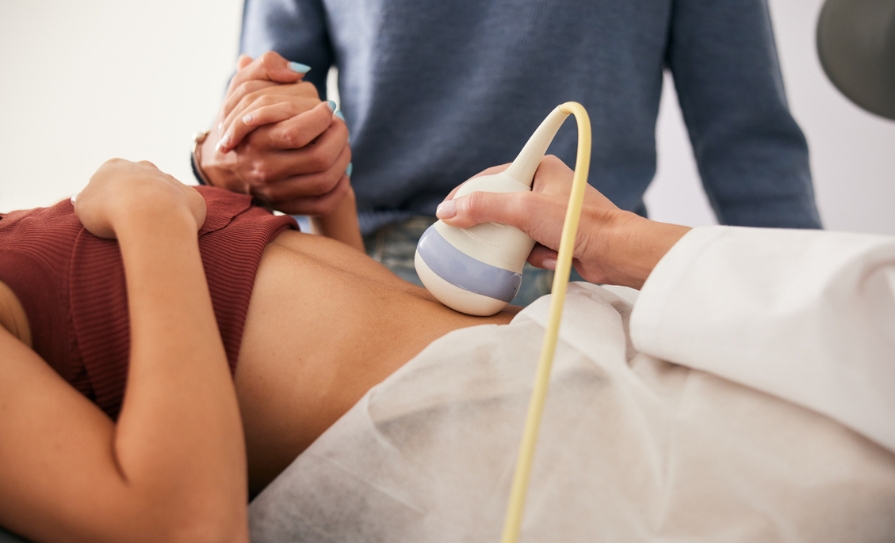
Publicly-funded in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI) was made available in Ireland in September 2023 to couples meeting certain criteria (ie, heterosexual couples, those using only their own sperm/eggs to conceive). Individuals must be referred through their GP to one of six local Regional Fertility Hubs.3 National Clinical Practice Guidelines3 for IVF and ICSI were published in March 2025. These include key recommendations for the indication, types of treatment, and standards of practice for assisted reproductive technology (ART) in Ireland. These guidelines are for healthcare staff or other professionals involved in the care of people with subfertility undergoing IVF or ICSI in particular. Before embarking on IVF or ICSI, performing ovarian reserve testing is important to allow clinicians to predict ovarian response and provide a more personalised treatment plan with the most appropriate treatment protocol and dosages. Female age, BMI, antral follicle count (AFC), anti-müllerian hormone (AMH), basal follicle stimulating hormone (FSH), oestradiol and inhibin B are all useful indicators. After age, both AFC and AMH have been found to be the most reliable markers of ovarian response. AFC is determined by counting the number of small, egg-containing follicles (2-to10mm in diameter) in the ovaries that can be seen on a transvaginal ultrasound. AMH is a hormone produced by the granulosa cells of ovarian follicles, the levels of which generally decline with age as the egg supply decreases. Semen analysis should be performed as a baseline investigation for the male partner. Issues with sperm concentration (oligozoospermia), motility issues (asthenozoospermia), or structure/shape (teratozoospermia) can affect male fertility. A full clinical history from each partner is also advised. Intrauterine insemination is less invasive and less costly than IVF, but it is less likely to result in pregnancy, particularly for older women. Standard IVF refers to adding sperm to oocytes, typically in a petri dish, to facilitate fertilisation. The sperm is required to swim to the egg and successfully penetrate the zona pellucida, leading to fertilisation. When semen analysis results are not favourable, intracytoplasmic sperm injection is favoured. ICSI involves the selection of a single sperm for injection into an oocyte. It does not improve pregnancy outcomes in nonmale factor infertility. IVF, compared to non-assisted conception, carries additional risks such as ovarian hyperstimulation syndrome (OHSS, Table 1), ADRs, VTE, and increased risk of multiple pregnancies.
2.1 births per woman5
| RISK FACTORS | SIGNS AND SYMPTOMS |
| Polycystic ovary syndrome | Rapid weight gain – more than 1 kg in 24 hours |
| High antral follicle count (>30) | Severe abdominal pain |
| High anti-Müllerian hormone (>30) | Severe, persistent nausea and vomiting |
| Age <35 years | Symptoms/signs of DVT – leg swelling, calf pain etc |
| Low BMI | Decreased urine output |
| Previous episodes of OHSS | Shortness of breath |
| High or steeply increasing serum oestradiol levels prior to trigger | Tight or enlarged abdomen |
Table 1: Ovarian hyperstimulation syndrome3
The in vitro fertilisation cycle
The steps in the treatment cycle are the same for both IVF/ICSI. The duration of a cycle depends on the stimulation protocol used, and the woman’s response. One ‘cycle’ or ‘round’ consists of the following:
1. Baseline ultrasound scan (including determination of AFC, as discussed above).
2. Pre-treatment medication: Pre-treatment (with oestrogen, progesterone or the COCP) aims to suppress LH/FSH secretion before the gonadotropin stimulation in the cycle. This can also help for scheduling purposes, allowing the clinics to plan IVF cycles.3 In cases where insulin sensitivity is an issue, such as in PCOS, pre-treatment with metformin may help increase ovulation likelihood. Insulin impacts fertility through multiple mechanisms, including through its role in ovarian steroidogenesis, and impact on ovarian follicle development.6
3. Controlled ovarian hyperstimulation (COH): Ovarian stimulation can be achieved in multiple ways, including administration of gonadotropins (ie, FSH), stimulation of endogenous FSH with clomiphene citrate, or administration of the aromatase inhibitor letrozole. The two basic protocols for COH are gonadotropin-releasing hormone (GnRH) antagonist and GnRH agonist.3 The protocols appear to have similar success rates, however, the antagonist protocol has been linked to a reduced incidence of OHSS. COH involves usually about 10- to-12 days of self-administered gonadotropins. A balance between insufficient and overstimulation needs to be reached to avoid OHSS. Higher doses of FSH are associated with an increased risk of OHSS, with no significant increase in the number of oocytes retrieved. While this treatment is ongoing, a premature surge in LH, which would result in ovulation, needs to be prevented. To achieve this, blocking/ downregulating GnRH is required. In the GnRH antagonist protocol, antagonists competitively block GnRH receptors, resulting in suppression of LH and FSH secretion by the pituitary gland. GnRH antagonists are started after initiation of COH with gonadotropins. In the normal physiological state, pulsatile secretion of GnRH from the hypothalamus leads to FSH and LH secretion from the pituitary. Administration of exogenous GnRH leads to initial hypersecretion of gonadotropin, followed by suppression, due to pituitary desensitisation. In the GnRH protocol, GnRH agonists are administered before starting COH.
1. Monitoring during stimulation: Monitoring of COH is important to identify OHSS risk, check ovarian response, and check endometrial response, using, ie, transvaginal pelvic ultrasound.5
2. Oocyte maturation trigger: Under usual circumstances, oocyte maturation occurs just before ovulation, and the oocyte is released from meiotic arrest (by LH), making it capable of fertilisation. In IVF, the trigger shot aims to ensure the same thing. A single injection of human chorionic gonadotropin (hCG), which has similar biologic activity to LH, is administered. In GnRH antagonist cycles, a bolus injection of a GnRH agonist (ie, buserelin) can be used, which more closely mimics the natural increase in LH/FSH, and is associated with a lower risk of OHSS. A combination of both can be used in some cases. If a fresh embryo (vs frozen) is planned, a hCG (as opposed to GnRH agonist) trigger should be given. The timing of this trigger is timed carefully to match the oocyte retrieval with the moment of peak egg maturity, usually given 36-to-38 hours prior to egg collection. The decision of timing should be individualised based on follicle size, duration of stimulation, organisational factors, and patient burden.
3. Oocyte retrieval (OCR): OCR is done using a needle and with the aid of ultrasound guidance, while the woman is under sedation.
4. Production of sperm sample/ thawing donor sperm.
5. Fertilisation and embryo development in the laboratory.
6. Embryo transfer: Under normal physiological circumstances after ovulation, the corpus luteum produces progesterone, which is essential for early pregnancy. It prepares the endometrium for implantation and secreting nutrients for the early embryo. In IVF, progesterone supplementation improves pregnancy outcomes. This should be prescribed for all women following oocyte retrieval (where the intention is to proceed with fresh embryo transfer). This should start no later than three days after egg retrieval, and continue at least until the pregnancy test is performed.5 NICE guidance recommends informing women that the evidence does not support continuing any form of treatment for luteal phase support beyond eight weeks’ gestation.2
7. Pregnancy testing. There are a range of regimens and medications for IVF. The National Clinical Practice Guidelines recommend that each clinic providing IVF/ICSI have their own SOP based on clinical evidence for prescribing medications. OHSS (Table 1) may occur in up to one-in-three cycles involving controlled ovarian stimulation with gonadotropins. It is a self-limiting condition with a spectrum of symptoms that occur as a result of increased vascular permeability. Vascular endothelial growth factor (VEGF) is found to be elevated in cases of OHSS. Cabergoline, the dopamine agonist, inhibits activation via the VEGF receptor and can prevent the increase in vascular permeability linked with OHSS, with studies finding that prophylactic cabergoline reduces the incidence of OHSS in high-risk women. VTE prophylaxis is indicated in women who develop OHSS.5 Infertility affects up to 12 per cent of couples globally, with age being the strongest predictor of female fertility. Assisted reproductive technologies like IVF and ICSI are increasingly used. National guidelines stress the importance of personalised treatment, ovarian reserve testing, and careful cycle monitoring to improve outcomes and minimise risks.
References
- Vander Borght M, & Wyns C (2018). Fertility and infertility: Definition and epidemiology. Clinical Biochemistry, 62, 2-10. https://www.sciencedirect. com/science/article/abs/pii/ S0009912018302200.
- NICE: Fertility problems: assessment and treatment (CG156) https://www.nice. org.uk/guidance/cg156/ resources/fertility-problemsassessment-and-treatmentpdf-35109634660549.
- Petch S, O’Byrne L, Hartigan L, Muresan B, Keneally J, Murphy C, McMenamin M. National Clinical Practice Guideline: In Vitro Fertilisation (IVF) and Intracytoplasmic sperm injection (ICSI). National Women and Infants Health Programme and The Institute of Obstetricians and Gynaecologists, March 2025 HSE https://www.hse. ie/eng/about/who/acutehospitals-division/womaninfants/clinical-guidelines/ in-vitro-fertilisation-ivf-andintracytoplasmic-sperminjection-icsi-2025-.pdf.
- Central Statistics Office. https://www.cso.ie/en/releasesandpublications/ep/p-vsys/ vitalstatisticsyearlysummary2024/#:~:text=The%20 fertility%20rate%20for%20 2024,it%20was%2030.5%20 in%202014.
- United Nations. World Fertility 2024. Available https://www. un.org/development/desa/pd/ sites/www.un.org.development. desa.pd/files/undesa_pd_2025_ wfr_2024_final.pdf.
- Holzer H, Casper R, & Tulandi T (2006). A new era in ovulation induction. Fertility and Sterility, 85(2), 277-284. https://www. sciencedirect.com/science/ article/pii/S0015028205038719.