Donna Cosgrove, PhD, takes a look at the management, treatment and diagnosis of infant pain and how the pharmacist can improve the quality of life for these patients
The modern thinking that pain is negative, and the aim to alleviate it with medical management, was not common in earlier times. In the 19th Century, pain was seen as useful in diagnosis and in treatment because it was associated with the body’s reaction to healing. Up until as recent as the 1980s, there was even a scepticism about the existence of infant pain.1 During this time, parent activism and paediatric pain research resulted in this view being challenged. Research indicates that infants experience pain similar to adults, possibly more intensely. This can lead to negative long-term consequences if the pain is not adequately managed.2 Noxious stimulation does not elicit the same pattern of activity in newborns as it does in adults. The immaturity of the synaptic circuits means that the infant pain experience is more diffuse and less spatially focused than in adults, and also under reduced endogenous control. This means it is potentially more powerful.3 Even now, paediatric pain is less understood, and less adequately managed, compared to that of older children and adults.4 Analgesic protocols designed for adults and older children cannot be scaled down for infants. This is due, not just to differing metabolism, but also because many pharmacological targets of analgesic medication are still developing and changing during infancy.3
Pain assessment in infants
In all patients, appropriate, frequent, and documented assessment of pain is vital to satisfactory pain control. Self- reporting of the patients’ pain levels is preferred due to the subjective nature of the experience, however, this is not possible in infants or non-communicative children. Additional assessment approaches, eg, pain assessment tools can help. The pain score from any assessment should not be viewed in isolation: Information such as family feedback, patient satisfaction, and physiological parameters also need to be considered.4 Infants have no language, so measuring pain expression in a reliable manner is a long recognised challenge. Assessment tools use combinations of behavioural measures, eg, leg movements and crying; physiological measures such as heart rate and oxygen saturation; and characteristic facial expressions of pain. An example of one of these, the FLACC pain assessment tool is shown in Table 1. It is a behavioural pain assessment scale used for nonverbal or preverbal patients who are unable to self-report their level of pain. No pain-to-mild pain is indicated by a score of zero-to-three, moderate-to-severe is indicated by a score of four-to-10.5
Untreated pain in infancy can lead to lasting consequences that persist into adulthood. This is because tissue injury in infancy may alter the normal course of development, leading to alterations in somatosensory processing and sensitivity to pain.3 Inadequate analgesia in infants leads to adverse perioperative outcomes, but this must be balanced with the risk of adverse effects with analgesic administration. Because controlled trials in infants and neonates are limited due to ethical and methodological constraints, published evidence of infant pain management is limited. Postop, procedural, and intensive care pain management in infants is mostly based on clinical experience.
Pharmacological management of infant pain
Immunisations (at two, four, six, 12, and 13 months in Ireland) are likely the most common painful events in infancy.2 Data suggests that as many as 95 per cent of babies will have been given paracetamol by the time they are nine months old.6 While paracetamol is universally recommended for use from neonatal life onwards, the maximum daily dose beyond this varies from 60mg/kg/day in New Zealand and the United Kingdom (UK) to 90mg/kg/day in the United States (US). Ibuprofen use in the US is only recommended from the age of six months, compared with three months in the UK and Ireland, and with a higher maximum daily dose compared to the UK and Ireland. Sugar free versions of paracetamol and ibuprofen preparations are preferable for infants because the ones that contain sugar are more damaging to teeth. Over-the-counter (OTC) paracetamol preparations are safe for children aged two months and older, and OTC ibuprofen is safe for children aged three months and older who weigh more than 5kg.7
Paracetamol has traditionally been recommended as a first-line treatment for fever and pain in children. Despite
the widespread use of these medications in a young paediatric population, some scientific literature cautions against the use of ibuprofen in, for example, young infants in terms of acute kidney injury risk, especially where the baby is dehydrated; and in primary
Paracetamol has traditionally been recommended as a first- line treatment for fever and pain in children
Varicella infection (chicken pox) where ibuprofen has been associated with a two- to-five-fold risk in the odds of developing a soft tissue infection. Growing evidence also suggests a link between paracetamol use in children and the development of asthma and atopic disease. Although there are studies investigating the risks of using paracetamol and ibuprofen in children, information about specific adverse effects on children under two, and particularly infants under six months, is sparse.
In terms of making the choice between ibuprofen and paracetamol in infants, studies comparing the safety and efficacy of ibuprofen and paracetamol show that ibuprofen is at least as effective in analgesia as paracetamol, and more efficacious as an antipyretic. No differences in safety between the two drugs were reported. However, the populations included in these studies were quite broad: Children aged from one month to 18 years, meaning that these conclusions were based on a more general paediatric population and may not be specifically applicable to infants and younger children.
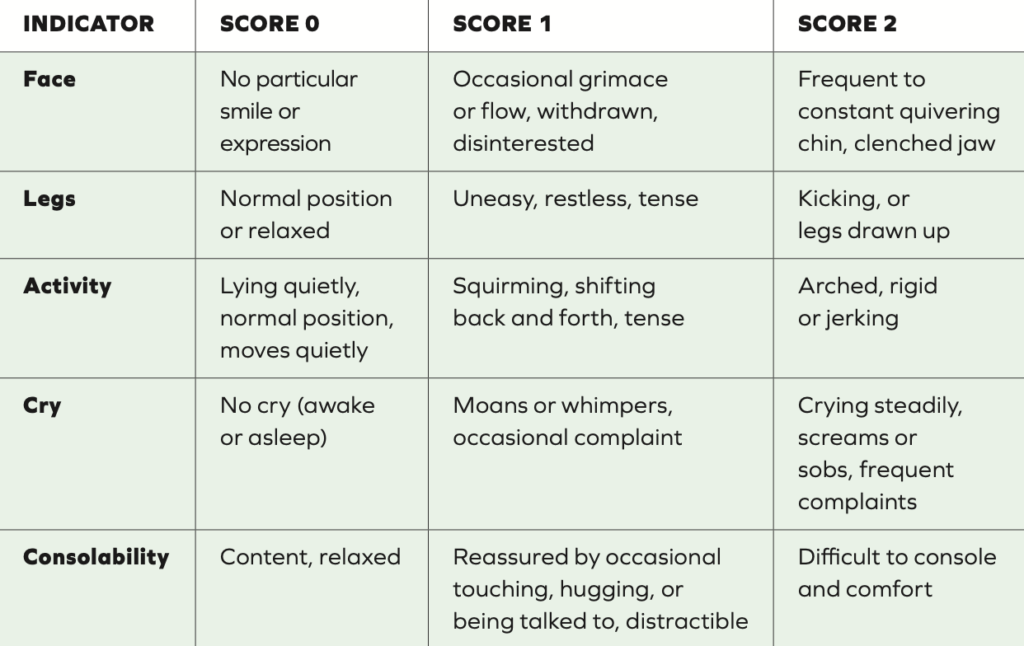
Table 1: FLACC pain assessment tool, suggested for two months to seven years. Each indicator category is scored from zero-to-two, giving a total score of zero-to-10 5
Authors of a 2020 meta-analysis identified 19 studies with data specifically available to compare the treatment of fever or pain with paracetamol or ibuprofen in children and infants under two years old. Compared with paracetamol, ibuprofen has a superior antipyretic effect at less than four hours and at four-to-24 hours (but
the superiority over paracetamol did not continue beyond 24 hours). Disappointingly, data for analgesia outcomes was not available for pain outcomes within four hours of treatment. Evidence suggests that ibuprofen shows a benefit over paracetamol in pain outcomes from four-to-24 hours.
In terms of safety, both drugs were found to have a similar safety profile in terms of serious adverse events, which were overall uncommon or rare. Most studies reported no adverse events. In this 2020 meta- analysis, authors conclude there was not enough information to compare the safety of paracetamol and ibuprofen in the under six months old cohort. In the only large scale RCT including infants younger than six months (The Boston Fever Study), which assessed the safety of ibuprofen for treating fever, none of the 319 infants aged one-to- six months were hospitalised for acute GI bleeding, acute kidney failure, asthma, or bronchiolitis, and risk of hospitalisation
did not matter by antipyretic choice.
No studies comparing ibuprofen and paracetamol and neonates are available so caution should be used when extrapolating results to this age group.
The authors of the review found no evidence to support the perceived risk of ibuprofen, or indeed paracetamol, causing toxic kidney effects in infants even with concomitant dehydration. There was insufficient evidence to support or refute the hypothesis that ibuprofen is responsible for an increased risk of serious bacterial infection in children (specifically invasive group A streptococcal skin infection in the context of primary Varicella infection). Results may be confounded because ibuprofen is usually used in more severe illness.6
Opioids are commonly used in the hospital setting in neonates and infants, with multimodal analgesia increasingly being used.3 World Health Organisation’s (WHO) guiding principles for the management of post-operative acute pain4 advises:
- Use of oral medication where possible;
- Administration of analgesics at
- regular intervals;
- Administration based on pain severity assessed by a pain intensity scale;
- Dosing tailored to the individual patient;
- Attention to detail throughout prescribing and administration.
Non-pharmacological management of infant pain
Non-pharmacological pain management options in older children and adults include massage, heat compresses, ice packs, repositioning, or physical activity as appropriate.4 Clearly non- pharmacological pain management measures are often not suitable for infants because they require a level of cognitive functioning not yet developed, for example, breathing training, relaxation, and imagery. In neonates and babies, sucrose, non-nutritive sucking and breastfeeding all reduce distress and the negative behavioural response to procedural interventions, for example, heel sticks, insertion of intravenous
lines, and nasogastric tubes.3 Parental presence, reassurance, and distraction are also useful to reduce distress. In a study examining the effects of distraction in infants aged two months to two years, distraction was observed to reduce distress associated with immunisation (injection). Infants were scored using the Measure of Adult and Infant Soothing and Distress (MAISD), a behavioural observation rating scale developed to evaluate the behaviours of infants, their parents, and nurses during painful paediatric medical procedures. Parents were instructed in distraction techniques for the child. During the immunisation, a movie (either Sesame Street or Teletubbies) was played. Parents were encouraged to redirect the infant’s attention to the movie (for example, ‘‘Big bird is singing you a song!’’ or ‘‘Look at that!’’). Infants showed low levels of “anticipatory” distress prior to injection, but clearly distress increased sharply during the injection. Infants at this stage of the immunisation procedure may not be receptive to distraction techniques. However, distraction was found to be very effective in decreasing distress after the injection during the recovery period, as opposed to a “typical care” condition where the infant’s distress actually increases in the minutes following the injection. Distraction techniques are a cost-effective, time-efficient, and easy- to-use intervention that can help comfort infants during these distressing visits.2
References
1. Rodkey EN, Riddell RP, 2013. The infancy of infant pain research: The experimental origins of infant pain denial. The Journal of Pain, 14(4), 338-350.
2. Cohen LL, MacLaren JE, Fortson BL, Friedman A, DeMore M, Lim CS, et al, 2006. Randomised clinical trial of distraction for infant immunisation pain. Pain, 125(1-2), 165-171.
3. Fitzgerald M, Walker SM, 2009. Infant pain management: A developmental neurobiological approach. Nature Clinical Practice Neurology, 5(1), 35-50.
4. Gai N, Naser B, Hanley J, Peliowski A, Hayes J, Aoyama K, 2020.
A practical guide to acute pain management in children. Journal of Anaesthesia, 34, 421-433.
5. National Health Service, 2021. Paediatric Clinical Practice Guideline: Management of acute pain in children. Available at: www.bsuh.nhs.uk/library/wp- content/uploads/sites/8/2021/07/ Paediatric-prescribing-guideline- Acute-Pain-Management-2021.pdf.
6. Tan E, Braithwaite I, McKinlay C J, Dalziel SR, 2020. Comparison of acetaminophen (paracetamol) with ibuprofen for treatment of fever or pain in children younger than two years: A systematic review and meta-analysis. JAMA network open, 3(10), e2022398-e2022398.
7. National Health Service, 2021. Medicines for babies and children. Available at: www.nhs.uk/conditions/ baby/health/medicines-for-babies- and-children/#:~:text=You%20 can%20give%20paracetamol%20 to,pharmacist%20before%20 giving%20them%20ibuprofen.