Dr Donna Cosgrove PhD MPSI examines the different options for contraception regimens
Introduction
The Report of the Working Group on Access to Contraception, published in 2019, estimates that in Ireland, 84 per cent of women use some type of contraception (although this number may have changed since then — this estimate is taken from a 2010 Irish Contraception and Crisis Pregnancy Study). In the UK, more than a third of women aged 16-to-44 years used oral contraception during the past year in 2010-2012.
Combined hormonal contraception (CHC) contains an estrogen and a progestogen delivered as a pill (COC), transdermal patch or vaginal ring. Current ‘low-dose’ COC was developed to reduce health risks associated with the high estrogen content of COC available in the 1960s and 1970s. CHC works by preventing ovulation: Effects on the hypothalamopituitary-ovarian axis suppress luteinising hormone and follicle-stimulating hormone, causing ovulation to be inhibited. Changes to cervical mucus, endometrium and tubal motility caused by progestogen exposure may also contribute to the contraceptive effect.
The majority of COC products are monophasic, ie, all pills in the pack have the same dose of oestrogen and progestogen, but multiphasic formulations are also available. Current evidence has not established whether multiphasic differs significantly from monophasic in terms of bleeding, side-effects, discontinuation rates or effectiveness. There is no clear advantage of using multiphasic preparations over monophasic, and as a result, monophasic regimens are recommended for first-line use.
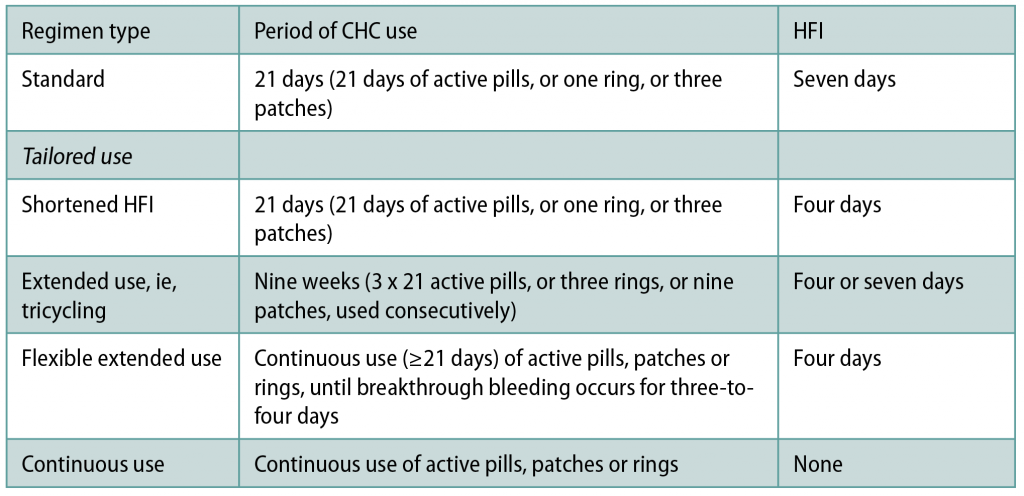
Tailored COC regimens
Most COCs are designed to be taken as 28-day cycles, with 21 consecutive daily active pills, followed by a seven-day hormone-free interval (HFI). The first seven pills inhibit ovulation and the subsequent 14 pills maintain this. These traditional 21/7 cycles were designed to imitate the naturally-occurring menstrual cycles, with a bleed each month. There is, however, no health benefit from a monthly withdrawal bleed: There are even some disadvantages. The withdrawal bleeding may be heavy, painful or unwanted, and the HFI is potentially linked to symptoms like headache and mood change.
Tailored regimens, which either reduce the frequency, shorten, or abolish the HFI altogether, can be safely used to avoid withdrawal bleeds and symptoms. They also theoretically reduce the risk of contraceptive failure. Less-frequent/shortened HFI that offers more continuous ovarian suppression could reduce the risk of escape ovulation that potentially contributes to contraceptive failure, particularly if CHC use is imperfect around the HFI. Although there is currently not conclusive evidence of greater contraceptive effectiveness, a standard seven-day HFI is associated in most studies with more hypothalamic-pituitary-ovarian axis activity than a shortened HFI. Ovarian activity is lower with continuous COC than with the traditional 21/7 regimens. Tailored regimens are as safe and as effective for contraception as standard 21/7 regimens — there is no health benefit linked to the traditional 21/7 CHC regimen with a monthly withdrawal bleed over other patterns of CHC use.
Data is currently too limited to recommend one tailored regimen over another. In some countries, COC that are intended to be taken as an 84/7 regimen with a three-monthly HFI are available. The Faculty of Sexual and Reproductive Healthcare (FSRH) supports off-label use of tailored CHC regimens (Table 1)using monophasic CHC that are licensed to be taken as a 21/7 regimen.
A Cochrane Review and additional observational studies have reported improvements in cyclical symptoms such as headache, bloating, tiredness and menstrual pain with extended COC regimens. In a group of 111 women trying the extended regimen, 80 per cent continued the extended regimen for the full year, and six months after that, most women reported that they had continued the extended regimen on their own. By using extended or continuous CHC, the frequency of withdrawal bleeds and associated symptoms (ie, headache, mood change) is reduced; this could be useful for women who have heavy or painful bleeding or problematic symptoms associated with the HFI.
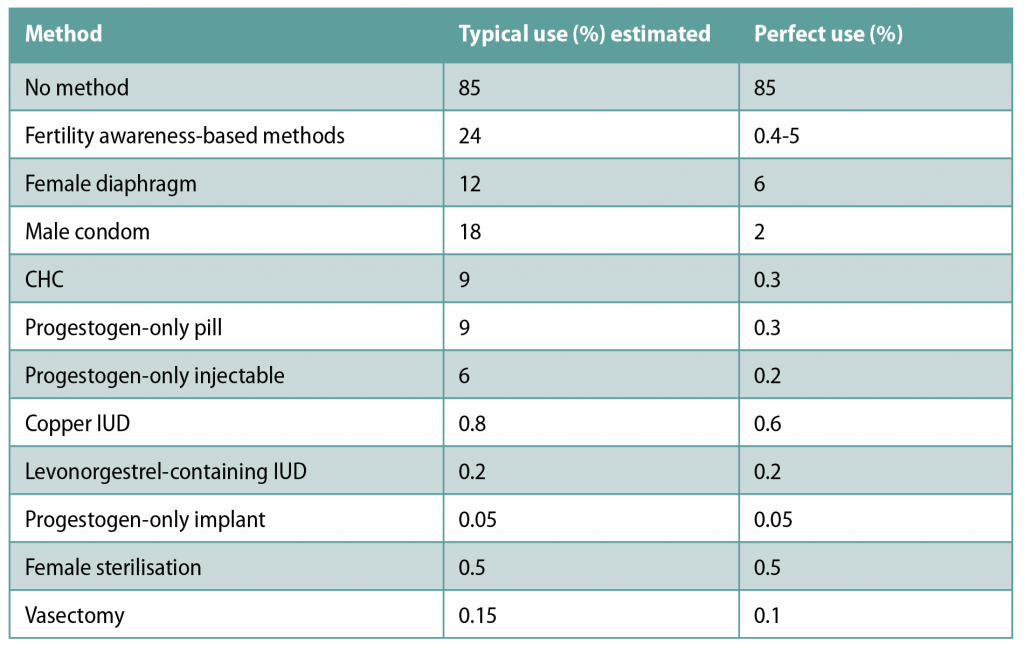
Benefits of CHC use
One advantage of using CHC is the relatively high efficacy. If used perfectly, the risk of contraceptive failure using CHC is just 0.3 per cent. Efficacy of these products is user-dependent, however — it is estimated that with typical use, 9 per cent of women have unplanned pregnancies in the first year of CHC use. Table 2 shows the percentages of women experiencing an unintended pregnancy in the first year of contraceptive use with typical vs perfect use.
Furthermore, there is no delay in return to fertility after stopping CHC, and no arbitrary maximum period of CHC use.
Additional benefits of CHC use include:
? A reduced risk of endometrial, ovarian and colorectal cancer.
? Predictable bleeding patterns.
? Reduction in menstrual bleeding and pain.
? Management of symptoms of polycystic ovary syndrome, endometriosis and premenstrual syndrome.
However, CHC use — particularly the HFI — is thought to be linked to side-effects such as mood change, headache and unscheduled bleeding.
Long-acting reversible contraception
Long-acting reversible contraception (LARC) methods are often an acceptable alternative. In a US study, 392 women aged 18-to-29 years were randomised to receive either a short-acting method or a LARC method of their choice free of charge. In the group randomised to LARC, acceptability and continuation rates were high, and unintended pregnancy rates significantly lower than for women randomised to short-acting methods. Women who frequently miss COCs or make repeated mistakes with COCs, patches, or rings should be advised to consider an alternative LARC contraceptive method that is less dependent on the user to be effective.
Attempts to disseminate ‘missed pill rules’ that reflect the evidence more accurately have concluded that simple rules are more likely to be followed. The resulting advice on what to do when pills (or other CHC methods) are not taken correctly is overly-cautious. A simple, over-cautious rule, however, is better than complicated rules that are less clear and not followed.
A COC pill is missed if it is not taken in the 24 hours after it should have been taken. Missing a single COC pill is not sufficient to reverse ovarian suppression, however, missing multiple pills, or extending the HFI by missing pills at either side, could theoretically increase risk of ovulation.
Precautions for combined oral contraceptive use
Drug interactions, particularly with hepatic enzyme-inducing drugs, and malabsorption, can impact the effectiveness of CHC. Hepatic enzyme-inducing drugs increase the metabolism of estrogens and progestogens, which could reduce the contraceptive effect of all CHC methods. Women using enzyme-inducing drugs should be advised to switch to a contraceptive method that is unaffected by enzyme-inducers (ie, intrauterine methods or the progestogen-only injectable). Most broad-spectrum antibiotics are not enzyme-inducing and no additional contraceptive precaution is required unless the antibiotics, or illness itself, cause vomiting or severe diarrhoea. Women taking lamotrigine should be advised that CHC may interact with lamotrigine; this could result in reduced seizure control or lamotrigine toxicity. Women using teratogenic medications should be encouraged to use the most effective LARC methods.
Limited evidence suggests a possible reduction in patch effectiveness in women ?90kg, although evidence relating to this is limited. In observational studies, height, weight and pregnancy are often self-reported, and potential confounding factors such as contraceptive compliance and frequency of intercourse are unknown. There is some evidence that ovarian activity in the HFI could be more pronounced in obese women, but most evidence suggests no association between weight/BMI and effectiveness of COC generally.
The increased risk of thromboembolism, breast cancer and cervical cancer associated with current or recent use of CHC is small, but is greater than with progestogen-only or non-hormonal contraception. COC containing higher ethinylestradiol (EE) doses may be associated with greater risk of arterial thrombotic events than lower EE doses.
Women should be informed about the health risks associated with use of CHC:
? Use of CHC is associated with increased risk of venous thromboembolism (VTE). Risk is highest in the months immediately after initiation, or when restarting after a break of at least one month: Frequent stopping and starting of CHC is therefore discouraged.
? Current use of CHC is associated with a small increase in breast cancer risk. This reduces with time after stopping CHC.
? Current use of CHC for more than five years is associated with a small increased risk of cervical cancer, with the risk again reducing over time after stopping.
Additional side-effects are also linked to CHC, but true association is unclear. Although headache is frequently reported as a side-effect of CHC, studies show that this also occurs with placebo. Unscheduled bleeding is a relatively common side-effect with CHC, but the incidence is not significantly higher than the background incidence of inter-menstrual bleeding reported. Some women may experience negative mood changes when taking CHC: There is not clear, consistent evidence, however, that CHC use causes depression. It has been suggested that mood change is common, and potentially related to external events. There is also no clear evidence that CHC use causes weight gain. Evidence on the potential effect of CHC on libido is mixed also. Women with severe hypertension (systolic pressure ?160mmHg or diastolic pressure ?100mmHg), or with BMI ?35kg/m2, generally should not use CHC, although CHC may be prescribed by a specialist.
Long-duration travel, not just air travel, is a weak risk factor for VTE. The risk increases with the duration of travel and with the presence of pre-existing risk factors for VTE. Data from prospective studies suggest an incidence of DVT of one-in-560 people travelling by air for eight hours. Retrospective studies suggest that PE is extremely rare in flights of <8 hours. Maintaining mobility during travel is a reasonable precaution, while for people without pre-existing risk factors, compression stockings and antiplatelet drugs such as aspirin are not indicated.
Women should be provided with written information or a link to a trusted online resource to support safe, effective CHC use, and specific signs/new diagnoses that should alert them to seek medical advice, such as:
? Calf pain, swelling and/or redness, or post-DVT or PE diagnosis.
? Chest pain and/or breathlessness and/or coughing-up blood.
? Loss of motor or sensory function.
? Breast lump, unilateral nipple discharge, new nipple inversion, change in breast skin.
? New-onset migraine.
? New-onset sensory or motor symptoms in the hour preceding onset of migraine.
? Persistent unscheduled vaginal bleeding.
? High blood pressure.
? High body mass index (>35 kg/m2).
? Blood clotting abnormality.
? Liver tumour.
? Symptomatic gallstones.







