Consultant in Addiction Psychiatry Dr Ciaran Somers analyses the potential advantages of an accelerated move to buprenorphine from methadone in pharmacies and GP clinics during Covid-19
In the context of the Covid-19 pandemic and difficulties encountered with supplying clients on opioid substitution therapy (OST) with their regular methadone dose, I present a case for the accelerated introduction of buprenorphine as an alternative to methadone OST in Ireland. As of September 2020, there were 376 clients prescribed buprenorphine in Ireland, as compared to almost 10,000 remaining on methadone. Factors in favour of buprenorphine include safer delivery, shorter duration of titration to a therapeutic dose, and a better side-effect profile relative to methadone.
Depot preparations of buprenorphine (buvidal) as weekly or monthly injections were approved by the European Union in November 2018 (European Medicines Agency, 2018), making it an even better alternative to methadone. I also present here evidence for the use of the opioid antagonist naltrexone as an alternative to OST. Psychotherapy and counselling, including motivational interviewing and 12-step programmes, remain an essential component in the management of opioid use disorders.
Opioids
The opioids include prescribed medications such as codeine, oxycodone, hydrocodone, methadone, buprenorphine and illicit opioids such as heroin, and other synthetic opioids, including fentanyl derivatives. Opioids are prescribed for analgesia, as cough suppressants and for the treatment of opioid addiction. Side-effects of the opioids, as a class, include sedation, respiratory depression, constipation, euphoria (in a minority) and as described here, reports of reduced testosterone levels associated with methadone. Both prescribed and illicit opioids can result in addictive use.
A recent heroin epidemic in the US (Centres for Disease Control, 2015) was contributed to by people who initially became addicted on opioid analgesics prescribed for pain, but switched to heroin when these analgesics increased in price relative to heroin. This epidemic in the US was also contributed to by increased regulatory control by government on prescribing of opioid analgesics. Pharmacotherapy for opioid addiction is primarily by opioid substitution with an agonist such as methadone or buprenorphine (partial agonist), or less effectively with an opioid antagonist such as naltrexone (McCance–Katz, E 2017).
Methadone was patented in 1941 in Germany as an analgesic. Buprenorphine, a synthetic derivative of the poppy plant alkaloid thebaine, was patented in 1961 and marketed in 1995 in response to an AIDS epidemic among heroin injection users.
Opioids’ mechanism of action
Methadone, along with heroin, oxycodone and hydrocodone, are all full agonists at the mu opioid receptor. They activate the receptor to full (100 per cent) activity and at increasing doses can cause respiratory depression, along with other adverse effects. By comparison, buprenorphine is a partial agonist at the same receptor, activating it to a maximum of 40 per cent of its potential activity. It is much more difficult to induce respiratory depression with buprenorphine due to this partial effect, also called a ‘ceiling effect’.
Caution, however, is necessary when buprenorphine is used in conjunction with benzodiazepines or alcohol, as this combination can result in respiratory depression. There also remains a risk of respiratory depression with buprenorphine in subjects with low opioid tolerance, such as children and others naive to its use. Buprenorphine also binds more tightly to the mu receptor than full agonists and when it is given to a client who has not been gradually detoxed from a full agonist such as methadone, the activity at the receptor drops suddenly from 100 per cent to 40 per cent, causing precipitated withdrawal symptoms.
For this reason, induction with buprenorphine requires careful monitoring for the presence of opioid withdrawal symptoms prior to administration. Withdrawal from full agonist compounds is necessary over days to a week prior to giving a first dose of buprenorphine (McCance-Katz E, 2017). In order to prevent diversion of prescribed buprenorphine for illicit intravenous use, it is administered in conjunction with naloxone as a product called suboxone.
When suboxone is taken orally, the buprenorphine component is absorbed sublingually, but the naloxone component is swallowed and inactivated in the lower gastrointestinal tract. When suboxone is diversified for injection purposes, the naloxone component is not inactivated and blocks the opioid receptor, preventing buprenorphine from binding to the opioid receptor and negating its effect.
Comparison of methadone and buprenorphine as OST
A comparison of methadone and buprenorphine opioid substitution treatment by Taylor et al (2019) stated that buprenorphine compared to methadone is:
- Associated with reduced mortality during titration and can be increased to a therapeutic dose (12-16mg) over days as compared to weeks.
- Has a milder withdrawal syndrome.
- At doses above 7mg, dropout rates from treatment are similar to those for methadone.
- May be administered every second or third day if given at higher doses (16-32mg/day) compared to daily administration for methadone.
- Is less subject to drug interactions. Plasma levels of methadone may alter when taken with drugs that inhibit/induce hepatic enzymes such as erythromycin, several SSRIs, antivirals (ribavirin) and some anticonvulsants.
Another potential advantage of buprenorphine is the relative absence of the stigma associated with methadone use. Buprenorphine is reported (Wedam et al, 2007) to increase the risk of cardiac arrhythmias (prolonged QT interval on ECG), but to a lesser extent than methadone. In favour of methadone, Mattick et al (2003) reported that subjects prescribed methadone described a faster onset of effect (‘rush’ or ‘buzz’) and preferred this medication to buprenorphine.
This latter report suggests that switching subjects, who had been prescribed methadone over prolonged periods, may lead to reluctance to comply or adhere to such a change in treatment regimen. Petitjean et al (2001) reported that buprenorphine was associated with serious headaches and less sedation compared to methadone. There is a reported enhanced risk with buprenorphine of diversion to intravenous use.
To reduce this risk, buprenorphine is administered in conjunction with naloxone as a product called suboxone (Taylor et al, 2019). When suboxone is taken orally, the buprenorphine component is absorbed sublingually, whereas the naloxone component is swallowed and inactivated in the lower gastrointestinal tract. When suboxone is diversified for illicit injection purposes, the naloxone component is not inactivated and blocks the opioid receptor, preventing buprenorphine from binding to the opioid receptor and negating its effect.
Hypogonadism associated with methadone use
Reduced testosterone levels are described for patients prescribed methadone. The normal testosterone level range in males is 270-1070ng/dl, with an average level of 679ng/dl. Bawor et al (2014) reported average testosterone level in men receiving methadone at 100ng/dl, compared to untreated at 414ng/dl. Chronic opioid prescribing as analgesia for both cancer and non-cancer related pain is reported to suppress the hypothalamic pituitary gonadal axis (hypogonadal state), resulting in secondary testosterone deficiency known as opioid induced androgen deficiency (OPIAD) (Ho K, 2019).
Persistently low testosterone levels were associated with adverse musculoskeletal metabolic and neuropsychiatric effects. These adverse opioid-induced effects began soon after administration, were dose-dependent and often remained following withdrawal of opioids. All forms of opioids may have this effect, but long-acting may be more harmful. Opioids with reduced mu receptor agonism (such as buprenorphine) may be protective.
Some hypogonadal symptoms were reported to improve with testosterone replacement and this treatment was recommended for symptomatic males with unequivocally low testosterone levels. Shadid and Barrett (2013) reported both sexes as being affected by hypogonadism induced by opioids. Symptoms in males included delayed ejaculation and erectile dysfunction and females experienced amenorrhea or oligomenorrhoea.
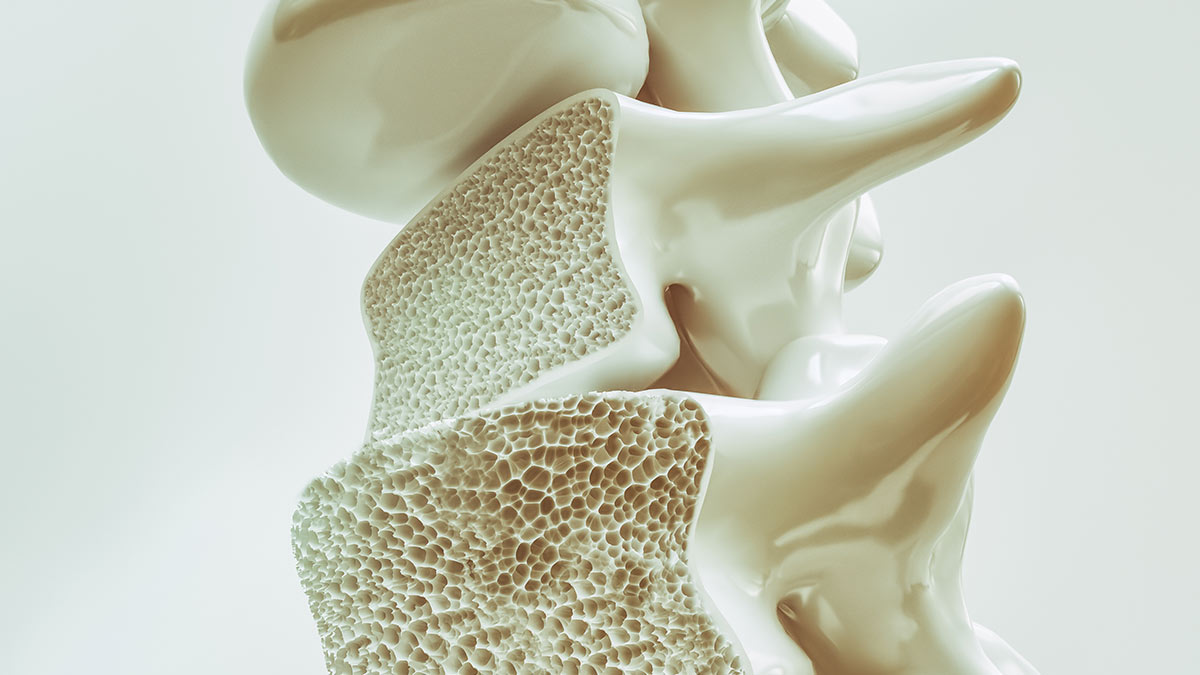
Hypogonadism was proposed to be a major risk factor for the development of osteoporosis. Grey et al (2010) reported male participants in a methadone maintenance programme (median 11 years’ duration) to have reduced bone mineral density (BMD) at lumbar, spine and hip sites compared to subjects not prescribed methadone. Serum testosterone was also significantly lower. They concluded that an assessment of skeletal health, including estimation of absolute fracture risk, should be undertaken in men participating in methadone maintenance programmes.
Studies have indicated that buprenorphine may have a less deleterious effect on testosterone levels than methadone. Bleisner et al (2003) compared plasma testosterone level and sexual function in men receiving buprenorphine and methadone compared to those on neither treatment. Subjects on buprenorphine had significantly higher testosterone levels at 510ng/dl compared to those on methadone 280ng/dl and a significantly lower frequency of sexual dysfunction. Testosterone levels of subjects on buprenorphine treatment did not differ significantly from those not on opioid medication, at 490ng/dl.
Comparative use of buprenorphine OST between countries
Relative use of methadone and buprenorphine for OST differs significantly between countries. Whereas in Ireland, the Netherlands, Estonia and Romania, buprenorphine prescribing as OST was less than 1 per cent, comparable figures for Wales were 30 per cent, France 60 per cent, and Greece 75 per cent (EMCDDA, 2019). In Scotland, methadone prescriptions decreased from 94 per cent of all OST in 2009 to 82 per cent in 2018. Suboxone increased from 4 per cent in 2009 to 14 per cent in 2015.
Buprenorphine-only prescriptions increased from 2-to-4 per cent in 2009 to 14 per cent in 2018 (Scottish Medicine Consortium, 2019). The NHS (Greater Glasgow and Clyde) made a decision to switch from suboxone to buprenorphine-only across all addiction services, including GP shared care in 2017. As the majority of their patients were given their medication under daily attendance (supervision), it was decided there was no need to include naloxone to prevent diversion (NHSGGC, 2017).
In France during the mid-1990s, access to buprenorphine was made widely available for those with opioid dependence. While the initial prescription and dispensing of methadone continues to be done in specialised addiction settings, buprenorphine can be commenced by any physician, including GPs, without the need for prior training or waiver. Similarly, buprenorphine can be dispensed by community pharmacists under the co-ordination of the prescriber.
Shadid and Barrett (2013) reported both sexes as being affected by hypogonadism induced by opioids
The maximum dose of buprenorphine approved per day in France is 24mg. During the first treatment year, the maximum prescription duration is longer for buprenorphine (28 days) compared to methadone (14 days), while urine screens are compulsory only for methadone. The introduction of this change has led to opioid dependence treatment in France becoming dominated by GP-based prescription of buprenorphine (60 per cent). This French public health harm-reduction strategy by opioid substitution therapy led to an 80 per cent reduction in opioid overdoses between 1994 and 2002.
There was also a fall in the incidence of HIV-acquired immune deficiency for IV drug users, from 25 per cent in mid ’90s, to 6 per cent in 2010. Despite the wide availability of buprenorphine among subjects with opioid dependence, the estimated rate of buprenorphine abuse remained stable at around 20 per cent of illicit opioid users, which is similar to the average for international data. It is estimated that now, 70 per cent of illicit opioid users in France have access to opioid maintenance treatment preparations, which corresponds to one of highest rates of treatment coverage in Europe (Polomeni and Schwan, 2014).
Eight other European countries, including Ireland, have a corresponding figure of over 50 per cent of illicit opioid users with access to opioid maintenance treatment (EMCDDA, 2019). On the wider international stage, reported figures for Australia in 2019 were 60 per cent prescribed methadone, 22.6 per cent suboxone, and 16.7 per cent buprenorphine only (Alcohol and Drug Foundation, Australia, 2020). Figures from 2015 for US federal government OST clients showed 356,843 in receipt of methadone, 21,628 on buprenorphine and 712 prescribed naltrexone (Alderks CE, 2017).
Buprenorphine/naloxone depot injection
The DEBUT trial, a randomised, controlled, open-label study of weekly and monthly depot buprenorphine (buvidal) compared to sublingual buprenorphine/naloxone tablets, was undertaken at six Australian sites (n=120). The study met the primary end-point, demonstrating a superior global satisfaction score (TSQM) for buvidal versus sublingual medication at week 24 (p=0.0143), as well as significantly higher effectiveness and convenience scores (p<0.0001). Patients treated with buvidal also reported significant improvements in quality of life, reduced burden of treatment, and other secondary outcomes versus daily buprenorphine/naloxone.
Retention in treatment with buvidal was high, with an 88 per cent retention rate at week 24 (Camarus press release, 11/2019). Woodruff et al (2019) have reported that depot buprenorphine constitutes a promising treatment option for some clients, including those discharged from prison or hospital and clinically-stabilised patients wishing to avoid daily oral PO medication, such as persons returning to employment. Buprenorphine s/c monthly injection was accepted for restricted use by NHS Scotland within a framework of medical, social and psychological treatment (Scottish Medicines Consortium, 2019). A commercially-sponsored Irish multi-centre clinical trial with buvidal is due to commence this December at two sites in Dublin — Domville House addiction clinic and the National Drug Treatment Centre.
Pharmaco-economics and regulatory environment
An Irish National Centre for Pharmaco-Economics evaluation of the economic effectiveness of buprenorphine was requested by the HSE in 2015. The evaluation failed to demonstrate a preferable cost-effectiveness for buprenorphine compared to methadone use in Ireland (INCPE, 2014). This finding remains relevant today, as buprenorphine continues to be a more expensive medication at point-of-sale.
A UK cost-effectiveness comparison of both methadone and buprenorphine against non-opioid substitution therapy (Kenworthy et al, 2017) showed that use of methadone and buprenorphine therapy respectively saved the NHS and Personal Social Services £14,200 and £13,900 sterling respectively per client/annum. When costs to society (including crime victim costs) were taken into account, the savings were £17,000 per client/annum for buprenorphine and £14,100 sterling for methadone.
Irish clinical guidelines for opioid substitution therapy (CGOST, 2016) primarily recommend continuation of methadone prescribing, but advise that buprenorphine/naloxone may be appropriate for some patient cohorts in certain circumstances. The exceptions listed included:
- Patients already receiving treatment with buprenorphine/naloxone.
- Those with a specific medical condition where methadone is contraindicated, ie, prolonged QT interval.
- Those naive to methadone, especially adolescents.
- Where detoxification is a primary goal of treatment.
- Where the main problem drug is codeine or another pharmaceutical opiate.
- Subjects whom the prescriber believes to be stable for at least six months, particularly in regard to employment or education, and committed to compliance with the treatment.
Based on these clinical guidelines, new Dáil legislation, the Misuse of Drugs Regulations 2017, was implemented in November 2017, which provides for prescribing of buprenorphine-containing products on the same statutory basis as for methadone. In response to a Dáil parliamentary question (Dáil Questions, PQ 37744/19), it was stated by the HSE National Clinical Lead in Addictions Services (letter dated 1/10/2019) that there had been a steady increase in the numbers of clients prescribed suboxone, from 117 in December 2018, to 240 in August 2019.
He explained that a phased introduction of buprenorphine throughout the country was extended to include all trained doctors working in HSE addiction services nationally and to all trained level 2 GPs working in community practice. The most up-to-date figure for suboxone prescribing for OST in Ireland was 376 in September 2020.
Naltrexone treatment
An alternative pharmaco-therapeutic treatment for opioid misuse is naltrexone, which is a mu opioid receptor antagonist. When it binds to the mu receptor, there is no activity (0 per cent), with no resultant analgesic, respiratory, euphoria or other opioid effects. For this reason, naltrexone should only be administered when a client has not received opioids for a week, otherwise, giving naltrexone blocks all activity at mu receptor, leading to significant withdrawal symptoms, as it displaces mu agonists from the receptor.
Naloxone, which is used for the treatment of opioid overdose, has a similar mechanism of action to naltrexone. Naltrexone (provides a complete 24-to-74 hour block of all opioid effects) is administered as PO 50mg daily or monthly injection 300mg IM. From a pharmacological perspective, naltrexone has been shown to be effective, but from an applied perspective, medication compliance and retention rates are poor. Mirozzi et al (2011) found no significant difference between all primary outcomes considered, in a comparison of PO naltrexone and untreated subjects on placebo.
The effect of naltrexone was found to be significant only where clients were forced to adhere to treatment, such as those in employment or on probation. Comparison by Lee et al (2017) of relapse prevention by naltrexone intramuscular injection against buprenorphine tablets found that initiation of naltrexone by subjects was more difficult, but once initiated, naltrexone and buprenorphine were equally safe and effective. Trials of naltrexone for alcohol dependence have given variable results.
Conclusions
In conclusion, there are many advantages for an accelerated move to buprenorphine from methadone, the present Covid-19 pandemic and associated methadone distribution problems being one. The primary advantage of buprenorphine is that it allows a more rapid induction and has a safer side-effect profile compared to methadone. Another advantage is that it is a more flexible OST relative to methadone, significantly reducing the need for daily intake.
Buprenorphine/naloxone tablets and buprenorphine depot injections dispensed/administered by GP clinics/pharmacies in the community would reduce the need for specific methadone clinics as at present. Stated preference by some patients for methadone, due to perceived euphoric effect and rapid onset, may create difficulties in switching them to buprenorphine. Male clients prescribed methadone should routinely have testosterone blood levels taken and where testosterone levels are significantly low, bone mineral density should be investigated. The use of naltrexone PO or depot injection provides an alternate strategy for managing opioid addictions and may also have a role in the management of alcohol addiction.l
References on request
Dr Ciaran Somers, Consultant in Addiction Psychiatry, Social Inclusion and Addiction Service, HSE Dublin North City and County, Ballymun, Dublin.