The Irish Skin Foundation (ISF) has called on HSE management to reimburse a first-in-class drug (dupilumab) to provide access for hundreds of people living with severe atopic eczema throughout Ireland. The ISF says that these patients, who have exhausted other treatment options, urgently need access to the new treatment, which has been available to patients elsewhere in Europe for over two years.
ISF board member and advocate Mr Paul Herriott, who has lived with eczema since childhood, explained: “Over many years, I have seen great changes in the medical management of my own eczema, from my earliest experiences with consultants in the North, to my various courses of treatment, wet-wrapping, phototherapy, and more.
“While the care I have received has been incredible and has allowed me to lead an essentially fully-active life, the burden of management has been utterly exhausting, and I eventually reached the limits of current treatments.
“This is the place where hundreds of patients living with severe and painful eczema now find themselves. I urge HSE management to allow people with eczema their fair share of access to new medications and a future without the ongoing ordeal and guesswork of daily management.”
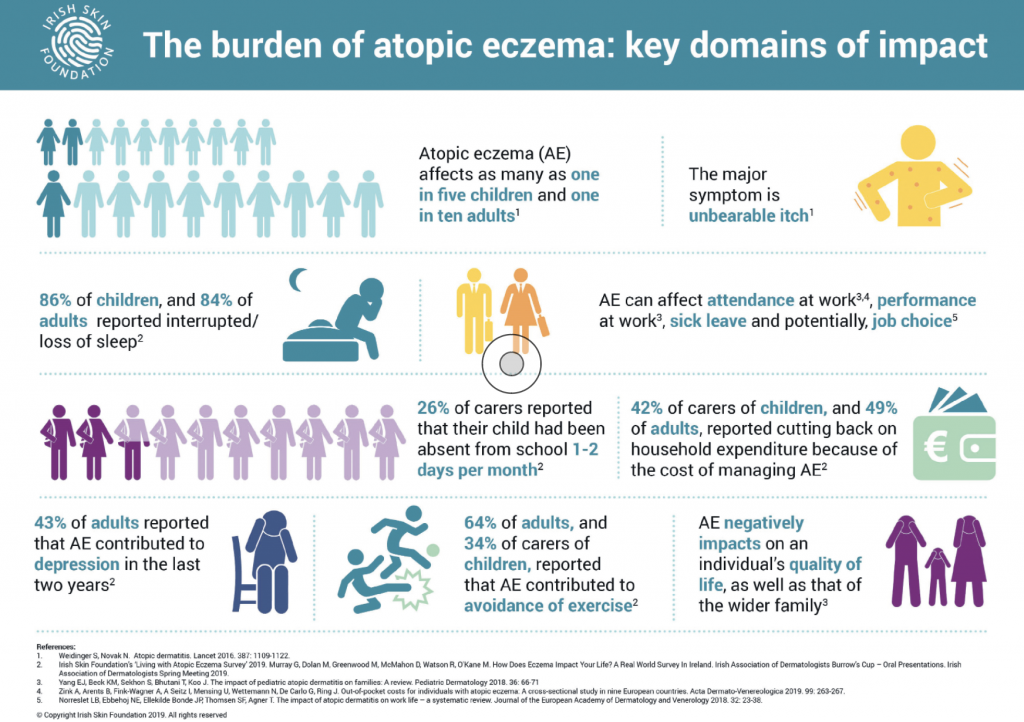
With very few treatment options currently available, managing moderate-to-severe atopic eczema can be complex and challenging, and remains the leading health burden attributable to skin disease, said the Foundation.
Prof Alan Irvine, Consultant Dermatologist at St James’s Hospital and Children’s Health Ireland at Crumlin, commented: “Irish patients with severe eczema have been waiting for approval for dupilumab for two years since the UK’s NHS made this often life-transforming drug available in the North. They and those who care for adolescents and children with eczema are anxiously awaiting parity of access across the island of Ireland.”
From the 14-country reference group, Ireland is among the very last countries (along with Greece) to provide patients with access to dupilumab. The new treatment has been routinely available in the UK to people living with severe eczema since August 2018 (including Northern Ireland).
“Many have been living their lives in a cruel limbo awaiting sign-off for this medicine. Healthcare systems across Europe have assessed cost benefits and made this drug available to their populations, following Germany’s lead in September 2017. Our patients are simply waiting too long now, their time has come,” Prof Irvine continued.